This is a post I’ve been meaning to write for some time, and is partly motivated by a discussion Drikan and I have been having on another blog. It will probably end up being two posts, with this one simply laying out the basic chemistry associated with the uptake of CO2 by the oceans. It will probably be a pretty dull post, so this is more for my benefit than anything else. I’m also not a chemist, so I don’t claim that I won’t make any mistakes. If anyone notices any, feel free to point them out.
The partial pressure of carbon dioxide, , in the atmosphere is related to the the concentration of
in the oceans,
, through Henry’s law
where is the Henry’s law constant. The units of
is typically mol/kg,
is typically atm – or ppm – and
is then atm/mol/kg. Henry’s law constant depends on Temperature and salinity, and at a temperature of
C, and salinity,
, of 34.78 g/kg, is 35.18 atm/mol/kg (or 0.02839 mol/kg/atm).
However, most of the dissolved inorganic carbon () in the oceans is not in the form of dissolved
. Dissolved
reacts with water,
, to form bicarbonate,
, and ionised hydrogen,
,
with a dissocation constant given by
Similarly, bicarbonate dissociates to form ionised hydrogen, and carbonic acid, ,
with a dissociation constant given by
Henry’s law constant, , and the two dissociation constants,
and
, depend on temperature and salinity and are given in this file. Given these constants, we now have 3 equations and 5 unknowns. There are, however, two other quantities that we need to know and that are also largely unaffected by changes in pH, pressure, temperature, or salinity. They are the
, and the titration alkalinity,
, given by
In surface waters, nitrate ions – – are an important nutrient and so their concentration is very low and can be ignored. Similarly, the contribution due to
and
, can also be neglected. We do, however, have to consider the dissociation of boric acid
with a dissociation constant
As with the other constants, also depends on temperature and salinity and is given in this file.
You may note that by adding this we’ve added another equation with two more unknowns. In the oceans, however, the residence time of boron is very long, depends on the Salinity, , and so the total amount of boron (
) is given by
which – if I’ve counted correctly – means we now have 7 equations with 7 unknowns.
To now determine the partial pressure of ,
, in equilibrium with the ocean, we first need values for the
and for
. Pre-industrial global averages for these two quantities are
, and
. The next step is to determine
. This is done iteratively by considering how the
and the carbon alkilinity,
, depends on
. From the equations for
and
we can write the DIC as
We can also use the equations and
to write
as
Dividing the equation for the by the equation for
gives a quadratic that can solved for
To determine , we simply make a first guess, then solve equation the equation above using
where we’ve used that
This process is repeated until the new value for matches the previous value, to within some small tolerance.
Once has been determined, we can determine
using
The partial pressure of ,
, is then given by
where is the inverse of Henry’s constant,
, and has units of mol/kg/atm. We’ve also converted
from
, to
. We can also determine the ocean pH from
.
That’s where I’m going to stop. Essentially, if you specify values for the dissolved inorganic carbon () the titration alkilinity (
) the salinity (
), and the temperature, you can determine
by first making a guess and then iterating to a solution. Given this, you can then determine the partial pressure of
in the atmosphere,
, and the pH of the ocean. What you can then do is see how various quantities vary with temperature or with DIC, which I will look at in the next post.
References:
Tans, P., Why Carbon Dioxide from Fossil Fuel Burning Won’t Go Away in MacAladay, J. (ed), Environmental Chemistry, Oxford University Press, 1998, pp. 271-291.
I also found this site useful, although I couldn’t get the code to actually run.
Eli Rabett, Quadratic coke.
Eli’s post about alkalinity.
A similar post by Nick Stokes.
Nick Stokes’s online calculator.

Needs some further proof-reading, ATTP. There are some weird mistakes.
Any hints?
The post is missing a story line.
Victor,
That’s certainly true 🙂 I’m planning a follow up (tomorrow if possible) that puts it into come context.
ATTP,
On a brief skim of the text;
cannot be correct. Bicarbonate dissociates to ionised hydrogen + carbonate ion (CO3)2-
Your next equation is wrong, but the equation for the dissociation constant (K2) is correct with Carbonate ion concentration in the numerator.
(NB. Trying out an html tag for subscript in this post, may not work …)
Phil,
Thanks. Fixed now, I think.
Thanks for topic. Bridge day today here but this is an important issue. Hope to get back with some comments/questions tonight.
One issue that needs addressing is the third side of the equation.
You have CO2 in the air, H2CO3/C02 in the ocean, but the third side is the substrate the ocean sits on.
Basically [sorry] any liquid with an earth base will end up having a pH that is a combination of the pH of the solution [water] and the pH of the earth base which dissolves into it.
My uninformed estimate* is that the substrate of the earth is basic,
Dikran may be able to help here, and that this would range from 7.9 to 8.5 in general.
This large substrate possibly has an immense buffering effect that prevents incidental acids like H2CO3 from ever altering the overall sea PH very much at all.
By an unconsidered negative feedback on the equations and their import above.
“possibly has an immense buffering effect… by an unconsidered negative feedback on the equations and their import above”
well aren’t we special
ATTP, sorry, was in a haste, Phil already said it.
angech, if only life was so easy. Have you considered the opposite, too? That the “earth substrate” actually has a positive feedback? If not, why not?
angech “This large substrate possibly has an immense buffering effect that prevents incidental acids like H2CO3 from ever altering the overall sea PH very much at all.”
and yet it has changed, which tells you something important about the scale of anthropogenic carbon emissions.
“By an unconsidered negative feedback on the equations and their import above.”
O.K. lets see your calculations (BTW it obviously isn’t unconstrained).
ATTP,
If I’m being “Nic Lewis” picky then
1. The text of previous correction still says “carbonic acid”, it should say “Carbonate ion” (the chemical formula after is now correct)
2. As you no doubt know, the square brackets [] are shorthand for concentrations. It is unconventional (to say the least) for these to appear in reaction coordinate equations (those with the double arrow ), which don’t say anything quantitative, but merely describe the species involved in the reaction. If this were for publication in a journal or text book, I would ask (or insist) for them to be removed so they follow the convention. Your second reference has this (largely) correct (their typo is to omit square brackets in the denominator of equation (3) for K2)
None of this influences your argument which, I note, is adapted from your second reference, and seems sound to me (My expertise is/was in gas phase spectroscopy, so I’m certainly not an expert in ocean chemistry).
Phil,
Thanks, I will correct that when I get a chance. I wasn’t sure about the square brackets, so put them in everywhere. I will correct it according to the convention.
Edit: This should now have been done, so – hopefully – the post now has the correct convention. Told you I wasn’t a chemist 🙂
Since angech is predictably trying to deny that increasing the partial pressure of atmospheric CO2 *will* alter ocean pH, there is of course solid evidence from palaeocean behaviour that it has done so in the past:
For a general overview, there’s Hönisch et al. (2012) The geological record of ocean acidification.
Specific to the PETM, there’s Penman et al. (2014) Rapid and sustained surface ocean acidification during the Paleocene-Eocene Thermal Maximum and Zachos et al. (2005)
Rapid acidification of the ocean during the Paleocene-Eocene Thermal Maximum.
Lots of evidence. Also see Fig 3 panel (c) from Zachos (2008):
You’ve essentially described the nuts and bolts of CO2sys.
http://cdiac.ornl.gov/oceans/co2rprt.html
Of the four parameters you need to characterize CO2 in seawater (Total Alkalinity TA, Total dissolved carbon TCO2, pH, and either fugacity CO2 or pCO2) only TA and TCO2 are independent of temperature and pressure. As long as you can measure pH and either pCO2 or fCO2 along with the temperature, salinity and pressure you can calculate TA and TCO2 or vice versa. The beauty is that we can measure Total Dissolved Carbon pretty easily and from there back out the rest of the carbon parameters (along with the CTD data)!
I just want to add that for all this focus on H+ concentrations the really important number is the saturation state (it’s the ratio between carbonate ions and carbonate concentration in seawater) as it also gives us information about the buffering capacity available.
( [Ca 2+] × [CO3 2-] ) / [CaCO3] = Ω where Ω>1 is precipitating and Ω<1 is dissolution.
Measuring the pH of seawater doesn't really tell us anything useful since dissolution of both forms of carbonate (aragonite and calcite) can occur far above a pH of 7. Furthermore, factors such as freshwater input which is low in alkalinity can push down saturation states without affecting pH much. We've seen this happen from both sea ice melt in the Arctic to glacial input in Southeast Alaska.
http://onlinelibrary.wiley.com/doi/10.1029/2008JC004862/full
http://www.sciencedirect.com/science/article/pii/S0272771414000663
angech,
As I understand it, buffering in ocean chemistry depends entirely on the presence of bicarbonate (HCO3-) and carbonate (CO32-) ions. As you add acid to a such a solution, the reaction
H30+ + CO32- H2O + HCO3-
is driven to the right, “mopping up” most but not all of the added protons. This continues until the carbonate concentration drops so that it can no longer perform that function. So given that when the buffer is in action, the pH is still changing (albeit slowly WRT added acid), the speciation diagram in ATTP’s 2nd reference shows that at pH of around 8 or less, the buffering effect of carbonate will be limited, and get less effective, since the concentration of carbonate is minimal. Carbonate buffering will be most effective at pH ~ 9 where there are reasonable concentrations of both carbonate and bicarbonate to “mop up” added or removed protons.
Dang it. On my blog I can add <sub> and <sup> HTML tags in comments and they do the right thing. Why don’t they work here … ?
Phil,
It should work, so I have no idea why they aren’t working here.
C Stoudt,
That was the idea. I was trying not to mess it up 🙂
Phil,
You might appreciate Jim’s Gaian chemistry.
More cobblers from Jim.
Meanwhile, in the real world, it may be that coral reefs won’t be around long enough to do that much more regulation of ocean chemistry: Veron et al. (2009) The coral reef crisis: The critical importance of <350 ppm CO2:
Willard:
I particularly liked this bit
which neglects to mention that living coral structures are also calcium carbonate which (unless the great god Gaia is looking over them) will dissolve equally along with the minerals …
The ‘Reverse Ouroboros’.
ATTP,
I wrote a post a while ago on solving these relations. I found that Newton-Raphson iteration of the relations worked pretty well. I put that into an online calculator which lets you do all sorts of experiments like adding fixed amounts of constituent. I’ve tried to emphasise the basic dependence on two variables, which can be chosen in various ways.
I also recommend de-emphasising the role of protons. It’s really a reaction between far more abundant Lewis acid/base species.
I think I mentioned in another thread that as ecosystems, carbonate reefs have appeared and been completely obliterated in the fossil record multiple times, each time with radically different reef building organisms… As a summary:
http://www.columbia.edu/itc/eeeb/baker/N0316/Lecture%205/page2.htm
It’s interesting because it is an example of multiple convergent evolution, and at the same time a fragile system that it is clearly possible to wipe out on a large scale.
Nick,
Thanks, I’ll have a look at that. I’ll think about what you say about de-emphasising the role of protons, but I have to admit to not fully understanding what you’re suggesting.
Marco says: October 31, 2016 at 6:21 am
“Have you considered the opposite, too? That the “earth substrate” actually has a positive feedback? If not, why not?”
My premise was that the materials making up the surface of the earth are slightly alkaline in nature with a pH around the 8 level causing the ocean to tend to this figure .[ the ocean is around 8.1].
No-one is disputing this.
This buffers any solution in contact towards this level.
In the case of adding an acid it would make the solution less acidic [negative].
It is hard to see how it could it more acidic.
–
BBD says: October 31, 2016 at 3:20 pm
“angech is predictably trying to deny that increasing the partial pressure of atmospheric CO2 *will* alter ocean pH”,
No.
ATTP has put forward a model explaining how CO2 increase will increase the acidity of seawater at the surface of the ocean. I agree with him.
It is useful and in fact can help crosscheck our discussion points. If the CO2 in air and sea match as suggested then temperature can possibly be worked out for both air and sea as well.
–
I point out that in considering the acidity of the ocean as a whole over time with increased CO2 that there are other factors to take into consideration.
A major one being the basic substrate which will negate both acidic and basic additions preventing any major deviation from the pH.
The pH reflects the pH of the liquid and its constituents.
There is a lot more earth in contact with the water than atmosphere and it has a lot more effect on the overall pH of the system.
The pH varies by up to 0.5 in different parts of the world ocean surface and varies by up to this amount seasonally in the one location and by smaller amounts in different parts of the same location but the average is around 8.1.
The same as the alkalinity of the earth surface on average.
–
“actual measurements of surface seawater chemistry show a predicted trend of a decrease of about 0.0015 pH units per year.”
– “the CO2 concentration in the surface ocean tracks the increasing values in the atmosphere, the penetration of that CO2 into deep water under a “business-as-usual” scenario of CO2 emissions, it will take about 500 years longer for a 0.3 unit decrease to occur in deep waters ”
–
“there is of course solid evidence from palaeocean behaviour that it has done so in the past:”
-“CONTEXT AND CONSTRAINTS FROM THE GEOLOGIC PAST On time scales of thousands of years and longer, the pH of the ocean is determined primarily by the cycling of CaCO3 (and some silicate) minerals.eventually, over thousands of years, changes in CaCO3 cycling will neutralize most of the excess acidity and restore the pH of the ocean to near-present-day value.”
–
angech says:
This buffers any solution in contact towards this level.
In the case of adding an acid it would make the solution less acidic [negative]*.
It is hard to see how it could it more acidic*.
–
[* Addendum Than if there was no buffer]
Obviously adding an acid makes a solution more acidic.
angech,
This isn’t my model. It’s essentially the model.
angech
Then explain how the PETM CO2 spike acidified the ocean (links above).
You have misunderstood Hönisch et al. The fact that “over thousands of years, changes in CaCO3 cycling will neutralize most of the excess acidity and restore the pH of the ocean to near-present-day value” is not in dispute. It has no bearing on the fact that if the change in CO2 partial pressure is rapid, it exceeds the ocean’s buffering capacity and ocean pH falls. This is what is happening now; it is what happened during the PETM and during other periods of rapid acidification. See eg. Clarkson et al. (2015) Ocean acidification and the Permo-Triassic Mass Extinction:
* * *
And please will you format your comments along conventional lines? What you write is often difficult to understand. It’s like reading porridge.
Sigh! Angech, I think you need to have the optics checked on your rose-colored glasses.
First, surface waters–where the acidification is occuring–are not in contact with the sea bed. And mixing of surface and deep waters is generally a pretty slow process (centuries). Second, what matters is not just the “pH” of the ocean floor, but the chemical potential of the solid and the solution. Now, as an exercise for the student, given that the abyssal waters have been in contact with the ocean floor for eons, how close to equilibrium do you think the two systems are?
The reason I suggest de-emphasising H⁺ is exemplified by irrelevant arguments about buffering. The main reaction here, which accounts for the quantities of species, is
CO₂+CO₃⁻⁻+H₂O⇌2HCO₃⁻
That is a Lewis acid-base reaction, and if CaCO₃₍ₛ₎ is present, may be supplemented by
Ca⁺⁺+CO₃⁻⁻⇌CaCO₃₍ₛ₎
By accounting for quantities, I mean that each mole of CO₂ reacts with and removes something like a mole of CO₃⁻⁻, and this is a measure of the progress of the reaction. H⁺ is a much stronger acid, and is present at all stages in very small concentration, which is determined by equilibrium relations with the more abundant species. Because of this dependence, pH varies slowly (which is the buffering). The small changes in [H⁺] do not produce significant changes in the concentrations of the main players, in terms of mass balance.
The importance of looking past H⁺ used to be greater, because as a sparse species, it was also hard to measure, and sensible people measured TA and DIC instead, and inferred [H⁺] when (and if) needed. This is somewhat less true now as better pH measuring devices become available.
“CONTEXT AND CONSTRAINTS FROM THE GEOLOGIC PAST”
It appears Angech stopped reading when the information was no longer to his liking. You know, the part where it says “Ocean acidification results from the fact that this natural oceanic CaCO3 cycle cannot keep up with the rapid rise in CO2.”
And also, later on:
“While glacial-interglacial cycles and hot house-ice house cycles provide information regarding the response of the ocean carbon cycle to changes in ocean pCO2 over thousands and millions of years, they are not good analogs to current acidification of the ocean by anthropogenic CO2.”
It’s amazing how Angech can find such relevant sources (“Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean”) and cherry pick the few sentences that fit his beliefs, ignoring everything written around it.
Seriously, Angech, read the whole report, and see how much we already know, and how worried we actually *should* be about the rapid rise in atmospheric CO2 for just this one reason of ocean acidification alone.
On this occasion, I provided the link upthread. It’s amazing that angech can misrepresent such relevant sources though. And a bit sad.
Angech has even confused me now. No, I didn’t link to the source he quoted and yes, just as you say – and quote – it contradicts him on the very next line.
Marco
Now I have a moment to look properly at this, I think angech has been very naughty indeed. Here’s angech’s quote with the lines he cut out restored (in bold):
That’s right, angech *cut* this from the quote he provided above:
That’s flat-out unacceptable IMO.
I agree with BBD, utterly unacceptable behaviour in a scientific discussion.
Nick, ,
,  , and
, and 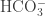 ?
?
Just to be clear, you’re suggesting that really we should combining the first two reaction equations I show in the post into s single equation relating
“really we should combining the first two reaction equations”
Yes. The point is that the reaction
H⁺+CO₃⁻⁻→HCO₃⁻
can’t proceed independently, because there are so few protons. It requires the simultaneous
CO₂+H₂O→HCO₃⁻+H⁺
to provide the protons. The reactions are coupled. Now it may be that H⁺ really does have a role in the mechanism, but if you are trying to represent that, there are probably many other intermediate steps. But if you want to focus on reactions with measurable inputs and outputs, it is better to combine them. It represents the real acid-base effect, which is the donation of an electron pair from CO₃⁻⁻ to CO₂.
And it bypasses the discussion of buffering, which confuses people. H⁺ buffering is mainly important if you are trying to follow the reaction by measuring H⁺, or pH.
BBD says: November 1, 2016 at 1:18 pm
“And please will you format your comments along conventional lines?”
I apologize for all grammatical and format problems, I hope you will forgive them when they occur, I do not mean to be unreadable.
Two different issues here.
One is ATTP and Nick discussing a formula for CO2 exchange from the atmosphere to the ocean and vice versa with resultant acidification of the surface layer of the ocean.
The second is a discussion on the rate and consequences of acidification of the ocean.
When discussing cherrypicking [guilty] I am dong no more than others here who discuss overall acidification but use examples of only surface acidification and do not specify the amount of the drop in pH hence cherrypicking as well.
My contention is that the pH of the ocean exists in a reasonably narrow band and that concerns re rapid and dangerous pH changes in response to increasing CO2 rely on more than a simple surface exchange mechanism.
The selective bits I quoted enhance the argument I put forward. They should not just be ignored.
We have a solution the sea in a crucible the land with an atmospheric interface.
What causes the present pH?
What is the current pH?
As Lucia would say, genuine questions.
What causes the pH to alter, and where?
Acidity is a difficult issue to grasp. The presence of carbonic acid in the blood and other body fluids helps control the pH level (acidity) of those fluids.
A change of 1 in the pH is a 10 fold change in the strength of the acid.
We have hydrochloric acid in the gut which dissolves most swallowed foodstuffs , yet it does not dissolve through our insides.This means that cells can adapt to and use different levels of pH.
Carbonic acid is a weak acid.
Carbon dioxide dissolved in water is in equilibrium with carbonic acid: CO2 + H2O ⇌ H2CO3
The equilibrium constant at 25°C is Kh= 1.70×10−3, which indicates that most of the carbon dioxide is not converted into carbonic acid and stays as CO2 molecules, the equilibrium is reached quite slowly.The rate constants are 0.039 s−1 for the forward reaction (CO2 + H2O → H2CO3) and 23 s−1 for the reverse reaction (H2CO3 → CO2 + H2O).
pure water (pH = 7)
Ocean water 8.1, so not the H2C03 effect then.
The pH of the ocean is due the minerals in the water?
These come from the substrate.
Increase the H2Co3 the base neutralises the acid and more base arise from the substrate.
Far more Substrate than CO2 so while a temporary small drop in pH will occur with increasing CO2 at the surface the overall effect for the whole oceanis far more subdued and always reversible.
angech,
You’re just asking question, despite there being answers to most of them. Once the CO2 has dissolved in the ocean, the amount of which can be dissolved is set by the Revelle factor (the oceans are expected to initially take up about 70-80% of our emissions over a period of hundreds of years) it is then further drawn down by reactions with calcium carbonate. This however takes thousands of years. The final drawdown is due to weather, which takes 100000 years. Overall, we would expect atmospheric CO2 to be enhanced by 10-20% for thousands of years and only return to pre-industrial levels in about 200000 years.
The chemistry is interesting and I think Nick makes a good point about the primary importance of the bicarb component. Angtech makes a specious one about the ocean floor sediments eventually neutralising any change.
The importance of the chemistry is in its impact on biological metabolism. The ocean carbon cycle dominates, and is in part driven by biological calcification. The white cliffs of Dover are not an inorganic deposit.
Some idea of the impact on oceanic ecosystems can be gleaned from areas with a local high CO2/low ph conditions. The problem is that this represents a small modification of a large general population and is still dependent on the larger ‘normal’ ecology.
Unfortunately the non-linearity in biological systems tend to be a Seneca cliff (TM Cassandra). one decade you have a struggling species, (coral, passenger pigeon) a decade later you have none.
angech wrote “The selective bits I quoted enhance the argument I put forward.”
However the bits you snipped directly still contradict your argument. Selecting the bits that enhance your argument and deliberately snipping the bits that contradict it is intellectually dishonest. Doubling down was not a good idea if you wanted to maintain some semblance of good-faith discussion.
I love this
Wonderful stuff. We can now solve any problem instantly. My suggestion is to remove any worries on rising oceans:
“Angtech makes a specious one about the ocean floor sediments eventually neutralising any change.”
Indeed, I’m no chemist or geologist, but I would have thought that one of the important things about sediments is that they form because the minerals cannot stay in (or enter) solution, which suggests that their interaction with the ocean is likely to be rather limited. Now if the ocean does become acidic enough to substantially react with the carbonate sediments on the ocean floor, then the same will be true of the carbonate tests, shells and exoskeletons of marine creatures, in which case angechs’ contention
“… the pH of the ocean exists in a reasonably narrow band and that concerns re rapid and dangerous pH changes in response to increasing CO2 rely on more than a simple surface exchange mechanism.”
would seem to have a bit of a flaw in the logic if ocean sediments are the underlying justification for this contention.
Pingback: Ocean CO2 uptake – part 2 | …and Then There's Physics
angech
Dealt with your trolling already upthread with numerous links to the literature.
It is established that a rapid increase in CO2 reduced ocean pH (bench chemistry). It is established that this is already happening (observations). It is established that it has happened before, repeatedly, in the past.
End of discussion.
Yes, angech is – in the midst of a huge Gish Gallop – simply asserting that acidification *shouldn’t* occur because he observes the ocean floor.
Flying in the face of the facts that we can observe that it does occur in controlled situations, has occurred in the oceans in the past, and is occurring now – as predicted.
Embarrassing.
angech should read the abstract of this paper.
Bit late, but angech and others have missed the point that the upper ocean which is in contact with the atmosphere is not bounded at the bottom by the bottom of the ocean, but by the lower ocean and the exchange between lower and upper oceans is very slow, and limited by the biological pump. Wrote about that a while ago.
Therefore angechs musing are well, not even approximate.
Eli good to see your comment , I think ATTP linked to your previous comments. Your reflection on boundaries and time and biological pumps is noted but not agreed with.
Trying to read paper ATTP, yes there are different time scales.
Rust never sleeps I have never asserted that acidification shouldn’t occur.
But don’ let that stop you asserting it.
BBD yes rapid increase in CO2 reduced surface ocean pH,I agree with you. As for reduced ocean pH you may have missed Eli’s late claim re upper ocean boundaries.
To be clear you should not use the term ocean when you mean surface ocean only.
ATTP’s work is on surface CO2, by extension it should spread to the whole of the ocean but it does not. My comments are directed at the pH of the whole ocean, not just the surface
Dikran
Sediments are a result of gravity and current flow and vegetation, rivers and rainfall.they usually form prior to becoming deposited, not because of the action of the solution. Most non organic sediment is made of the minerals found in the earth’s crust which are slightly basic.
Your suggestion that a substance’s interaction is likely to be limited because it is not dissolving or being precipitated ie as a sediment is misleading. It has interacted already over millions of years to reach the state it is currently in.
You have natural water , pH 7 yet the ocean is pH 8.1.
Precisely because the earth it is in contact with has caused the pH of 2.4 percent of the earth by mass,the total ocean, not just Eli’s boundary surface level, has been forced to go up 1.1 in pH.
It would be interesting to work out how much acid would be needed to change the pH of the whole ocean by 0.1 down from 8.1
Even more interesting is the log basis of pH , not mentioned often here which means each successive drop in pH requires a lot more acid than the initial drop.
And that CO2 is a weak acid so after a certain amount of dropping one would need enormous increases in CO2 levels to reach enough acidity to cause a further drop and the effect would peter out well before approaching the pH of H2CO3.
angech, if you believe so much in your “earth’s crust” buffering, can you explain the first map shown in the link below?
https://www.researchgate.net/publication/253469151_Direct_observations_of_basin-wide_acidification_of_the_North_Pacific_Ocean
The deeper down you go, the lower the pH, and the more north you go, the lower the pH. Explain!
I won’t recommend you read the paper, because you’ll likely cherry pick a sentence from it that suits you, and ignore all the rest. You already show that by requiring us to focus on the whole ocean, thus ignoring that the flora and fauna of the oceans are primarily present in this “surface” region (primarily the euphotic region, down to about 200 m).
angech
I do not mean surface ocean only because the pH shift is not confined to the surface layer only. You should learn about the vertical structure of the oceans and the exchange between the surface, mixed and upper ocean layers. RTFRs, please.
angech, if you think I am going to respond to yet another of your Gish gallops after your dishonest quotation exposed above, you couldn’t be more wrong. This is especially true as your comment did not even address the contradiction in your argument that I pointed out. Give it a rest angech, nobody is impressed by your sophistry.
Notice that a study like Penman et al. (2014) can be titled Rapid and sustained surface ocean acidification during the Paleocene-Eocene Thermal Maximum yet contain the following statement (my emphasis):
Read more, deny less.
@ Dikran
I’m mighty pissed off with that particular bit of dishonesty too. I should follow my own resolve.
@BBD sadly this is not the first time my patience has run out due to angech’s
disingenuousness (see here and follow the links back for a previous example). Sadly angech shows no sigh of addressing his behaviour (arguably the misrepresentation by selective quoting on this thread was if anything worse).
Marco,
The paper you quoted gave the information you asked for.
Part 3 results and discussion
Lower temperature allows greater CO2 uptake, resulting in lower pH.
This might explain the lower pH as one goes north.
As for pH decreasing with depth this is not right there is an increase in CO2 due to biological causes in depth from the surface for the first 200 to 1000 meters (cold water poles/hot water tropics) reasons but once one runs out of enough oxygen near the surface the pH rises slightly.
The paper states, no cherry picking, that pH changes were essentially zero below 800 meters for the time period studied though it does state that such changes would be expected over greater time frames.
Of interest the study has numerous assumptions and impressive accuracy in its measurements of pH to a level of 0.017 per year despite the pH levels being measured varying in range over a 0.7 range.
angech is being intellectually dishonest again:
“The paper states, no cherry picking, that pH changes were essentially zero below 800 meters for the time period studied though it does state that such changes would be expected over greater time frames.” [emphasis mine]
This is a red herring because of course nobody would expect there to have been a big change because the ocean is strongly stratified, so it is an entirely irrelevant point. It is a huge cherry pick because he mentions this irrelevant point, but not:
“On time scales of several centuries, dominant controls on seawater pH include atmospheric CO2 exchange and the oxidation of dissolved and particulate organic matter in the water column”
Note that angech’s “substrate the ocean sits on” influence is not mentioned at all, so it hardly supports angech;s argument. Also the abstract says:
“In the surface mixed layer (depths to ~100m [DM i.e. where most coral exists, for example]), the extent of pH change is consistent with that expected under conditions of seawater/atmosphere equilibriation, with an average rate of change of -0.0017/yr. Future mixed layer changes can be expected to closely mirror changes in atmospheric CO2, with surface seawater pH continuing to fall as atmospheric CO2 rises”
In other words the mixed layer pH is changing in response to changes in atmospheric CO2, which directly contradicts angechs contention that:
… that the pH of the ocean exists in a reasonably narrow band and that concerns re rapid and dangerous pH changes in response to increasing CO2 rely on more than a simple surface exchange mechanism.
The “dangerous” pH changes are the ones in the mixed layer where e.g. corals are found, and in the mixed layer there is no evidence that pH changes depend on anything more that simple surface exchanges.
Very shabby indeed, and fools nobody.
Also angech’s reply dodges the question Marco actually asked, angech was arguing that the ocean substrate neutralises acidity in the ocean, if that were true the deep ocean would have less acidity, rather than more than the surface ocean as it is the deep ocean that is in contact with the sea floor. So where is the evidence of this effect in the observations mentioned in the paper? Nowhere as far as I can see. Where was angech’s admission that the paper provided no evidence to support his argument? Nowhere as far as I can see.
Funnily enough the pH of the deep ocean is less acidic as it approaches the ocean floor and is less acid in contact with the sea floor. Look at any charts with depth pH.
Look at the charts that are in the article Marco linked to.
Click on his link, it is all there, the evidence that you claim you want.
Sorry, I realise that you did click on it by your comments but may have inadvertently missed the second depth chart, the one that goes below 1000 meters down to 6000 meters.
The alkalinity of the ocean, 8.1 by ATTP’s chart in the part 2 discussion is certainly not caused by H2CO3, it is an acid. CaCO3 leaches into the sea from the crust and saturates the ocean and causes it to be alkaline.
Therefor it has to be more basic closer to the crust*.
Causes of increased acidity at the surface are CO2 and organic activity or to be more correct organic breakdown which also causes some decrease in pH in the shallow oceans down to 1000 meters first depth chart that causes the confusion.
angech,
But it is not less acidic than the surface layers.
“Funnily enough the pH of the deep ocean is less acidic as it approaches the ocean floor and is less acid in contact with the sea floor.” ?????
http://www.skepticalscience.com/print.php?n=918
First figure, it is *more* acidic as you approach the ocean floor!
“Of interest the study has numerous assumptions and impressive accuracy in its measurements of pH to a level of 0.017 per year despite the pH levels being measured varying in range over a 0.7 range.”
Welcome to the world of statistics. Perhaps Dikran can make an attempt to teach you something about that, but I think he will pass.
ATTP,
Astute comment.
The reasons for the variation at depth and and latitude are complex and I hope my answers are both scientific and correct.
Marco’s paper has all the answers for his questions.
Lower surface temps as one goes polewards decreases pH is due to more CO2 being able to dissolve in colder water.
The deeper down you go the lower the pH is not correct as I explained above, the buffering effect is very noticeable as one approachs the deep earth crust.
The high surface pH is due to higher temperatures at the very surface layers causing CO2 to be expelled hence highest pH always at the surface. Both the colder water and organic activity cause a decrease in pH over the first 200 to 1000 meters.
After that the temperature of deep water, 100 to 6000 meters hardly varies and the CO2 becomes increasingly neutralised by the CaCO3 resulting in the pH increase.
I think it is more a question of evolution/biology than stats (and a bit of maths). 0.017 per year is 0.17 per decade and 1.7 per century, added to which pH is a logarithmic scale, rather than a linear one, so just because a rate is numerically small, does not mean that it isn’t something problematic. As to the range, different organisms evolve to suit the environment in which they occur. Just because there are variations in ocean pH of 0.7 (it isn’t clear whether that is spatial or temporal), does not mean that all marine life can live in those conditions, or that the range isn’t already at the limit of what an organism can live with (organisms tend to adapt to conditions and expand to fill the available habitat that is suited to their adaptions). Thus if you alter the pH of the oceans, you are likely to endanger populations that are at the limit of their range.
This is a bit like saying that CO2 is rising at only a couple of ppm per year, so it can’t possibly have any negative effect, and about as valid.
The funny thing is that angech keeps going on about CaCO3 neutralising the acid. What does he think the shells of marine life are made of?
I also don’t think it is true that CaCO3 leaches into the sea from the crust and saturates the ocean and causes it to be alkaline.”, at least if he means from the sea floor. My intuition suggests that the sea floor is mostly covered in organic mud, which is likely to insulate the ocean from the sediments below. My understanding is that the CaCO3 mostly comes from biological sources in the surface waters and run off from rivers etc, but I might be wrong. In any case, it has been pointed out to angech repeatedly that this is not a rapidly renewable resource, and it takes thousands of years to bring down atmospheric CO2.
angech wrote “The deeper down you go the lower the pH is not correct as I explained above, the buffering effect is very noticeable as one approachs the deep earth crust.”
The observations say otherwise.
angech,
Maybe you can clarify your actual point. It is clear that an enhancment in atmospheric CO2 will decay and one way it will do so is via reactions with CaCO3. However, the key point is that the timescale over which atmospheric concentrations will return to pre-industrial levels is > 100000 years. Do you dispute this?
“However, the key point is that the timescale over which atmospheric concentrations will return to pre-industrial levels is > 100000 years. Do you dispute this?”
an enhancement in atmospheric CO2 can return to pre industrial levels over many timescales and at different timescales. Factors include the amount of enhancement and the temperature of the ocean and the rate at which CO2 is neutralized and removed from the system.
Large amounts being removed solely by the mechanisms you refer and under the conditions you stipulate could take > 100000 years.
The contention is that the supersaturated CaCO3 in the ocean, the cause of it’s pH being 8.1,
exists in large enough amounts to neutralize the relatively small amount of excess CO2 in a much shorter time than your source credits.
Note it only talks of neutralization at the sea floor whereas we both know that this process is happening throughout the whole ocean. 5-8% at the seafloor is a lot more if the whole of the ocean is taken into account and a lot quicker in time.
The CO2 of 280 ppm did not make the ocean acid, nor will 400 ppm of CO2 or 1600 ppm, It will reduce the surface pH while it remains in excess but the eventual level of CO2 when the excess is removed is a natural function of the amount of CaCO3 in the ocean, which is not atmospheric in origin.
If excess CO2 is put into the atmosphere it is neutralized, buffered, eliminated but the supply of CaCO3 is inexhaustible.
Dikran Marsupial says:
November 7, 2016 at 12:51 pm
“angech wrote “ the buffering effect is very noticeable as one approaches the deep earth crust.
The observations say otherwise.”
The graph of pH [thanks] for 1000 to 6000 meters agrees perfectly with my comment.
PH increases with depth, noticeably.
The apparent decrease with depth for the shallow levels is due to temperature reduction, higher CO2 concentration with lower temperature not due to the depth per se. In other words if the temperature of a column of water was the same pH would become more alkaline with depth as it approached the higher concentrations of CaCO3 at the sea floor.
This is scientifically correct and deserves acknowledgment as such.
“The apparent decrease with depth for the shallow levels is due to temperature reduction”
Actually, it is due to CO2 production as a result of organic matter oxidation.
“In other words if the temperature of a column of water was the same pH would become more alkaline with depth as it approached the higher concentrations of CaCO3 at the sea floor”
Depending on the ocean, the CCD is between 4000 and 5000 meters. This means that at this depth there exists no solid calcium carbonate. Thus, there cannot be a dissolution of calcium carbonate from the deep ocean floor, because it does not exist on the deep ocean floor. If anything the carbonate comes from ocean plankton that drops to below the CCD. Note that the CCD will rise with increasing CO2 in the atmosphere, most directly observed at regions of downwelling. In upwelling regions, the CCD is in a way set by the CO2 concentration and temperature of the old atmosphere of tens of thousands of years ago.
You’re dodging the question. Do you dispute that an enhancement in atmospheric CO2 will take > 100000 years to return to pre-industrial levels. Just for clarity, let’s also say an enhancement of at least hundreds of ppm.
angech “The apparent decrease with depth for the shallow levels is due to temperature reduction, higher CO2 concentration with lower temperature not due to the depth per se. In other words if the temperature of a column of water was the same pH would become more alkaline with depth as it approached the higher concentrations of CaCO3 at the sea floor.”
I don’t think you have the causal relationship right. As I understand it the CaCO3 in the deep ocean is largely the result of the biological pump, i.e. the dissolution of the carbonate tests and shells of marine organisms that drift down from the surface waters, as Marco explains. Now I am not claiming that there is no transfer of CaCO3 from the ocean floor to the deep ocean, but my understanding is that there isn’t very much. BTW note that sedimentary rocks such as limestone tend not to form in the deep ocean because of the dissolution process that Marco mentions, so it isn’t clear that there is an abundence of CaCO3 in the rocks forming the ocean floor to begin with (I suspect the Atlantic ocean floor is mostly baslt, i.e. silicate rock).
So what is your evidence for an unlimited supply of CaCO3 from the ocean floor?
angech wrote “This is scientifically correct and deserves acknowledgment as such.”
angech, on the other thread you admitted that you didn’t really understand the nature of acidity. What makes you think you are in a position to say what is scientifically correct, especially when the scientists who actually work on this disagree with you. I’m not saying that I am either, but then I don’t have the hubris to state that the experts are wrong.
I think it is worth quoting this bit from Archer et al. (2009)
I do not get the intransigence on the issue of pH at various depths in the ocean and the need to argue about issues that I raise when they are factually correct.
If one insists that decreasing ocean acidity must be acute, extreme, dangerous to all marine life and about to happen now then I get that any evidence to the contrary might be uncomfortable.
I would hope that someone here has some oceanography background and is prepared to stick his neck out on the issues of ocean pH and alkalinity and confirm the facts I have mentioned.
Dikran,
I realise that our views are opposites and that arguing with you does not make my views more palatable (but).
The top of the ocean is more alkaline than the next 1000 meters despite being closest to the source of extra acidity for the ocean (atmospheric CO2).
Only decent explanation is there is more dissolved CO2 in colder water.
The causal relationship is right. There are bucket loads of carbonate in the earth’s crust, every subsurface volcanic eruption exposes more to the ocean. The ocean is alkaline not because of current biological deposition but from leaching from the earth over billions of years.
The ocean is alkaline from necessity, it determines most of the CO2 level in the air, not the other way round precisely because of ATTP’ s formulae.
“I do not get the intransigence”
i.can’t.stop.laughing
angech,
Who has insisted this. You appear to be doing the standard of assuming things about what others are saying despite them never having said anything close to what they’ve assumed.
You still haven’t answered my question. You might also want to read what I quote here.
angech wrote “If one insists that decreasing ocean acidity must be acute, extreme, dangerous to all marine life…
More disingenuous behaviour from angech, it has been repeatedly pointed out that the major danger to marine ecosystems is in the surface waters, such as coral reefs etc. It has also been pointed out that nobody expects a rapid change in acidity in the deep ocean, yet angech is arguing that there is no danger on the grounds that deep ocean acidity is essentially unchanged.
Note also the usual hyperbole of “extreme” etc. just like CAGW is generally something said by skeptics, rather than mainstream people as a way of avoiding doing anything. It doesn’t need to be extremely dangerous for marine ecosystems to justify doing something about it.
The top of the ocean is more alkaline than the next 1000 meters despite being closest to the source of extra acidity for the ocean (atmospheric CO2). Only decent explanation is there is more dissolved CO2 in colder water.
angech, you are missing the point. Marine ecosystems adapt to the environment in which they evolved. The problem is not the acidity, it is the change in acidity. This aspect of ocean chemistry is well understood as Marco and I have pointed out to you, but it doesn’t mean that chemical erosion of the sea floor is the primary driver.
There are bucket loads of carbonate in the earth’s crust, every subsurface volcanic eruption exposes more to the ocean.
I don’t think this is necessarily correct. I would have thought that the lava from mid-oceanic volcanoes is largely basalt (silicate). As I understand it, the reason that land based volcanoes give of a lot of CO2 is because they are largely erupting magma formed from subducted crust from shallower oceans that has carbonate sediments. Even then, as both I and Archer have pointed out, a layer of organic mud quickly seals the ocean floor, limiting further chemical erosion, a point you have ignored yet again.
“The ocean is alkaline not because of current biological deposition but from leaching from the earth over billions of years.
I asked you for your evidence of this, not an assertion (which disagrees with Archer, who has actually studied this). So where is it?
Just as Dikran says, but to be more precise, the problem is the rapidity of the pH shift. When organisms cannot adapt fast enough to keep pace with environmental, they tend to die out.
That should be “environmental change“.
Bad day.
Dikran Marsupial says: November 9, 2016 at 9:56 am
” I would have thought that the lava from mid-oceanic volcanoes is largely basalt (silicate).”
Very true.
Does not alter the fact that ” There are bucket loads of carbonate in the earth’s crust, every subsurface volcanic eruption exposes more to the ocean.”
You agreed to the CO2 release from heating and disruption of the carbonates in the earth’s crust by volcanic events on the seafloor in this comment,
“BBD says: October 12, 2016 at 7:11 am
You can see the towering hyperthermal of the PETM at ~55Ma. Here in close-up, you see the ~200ka recovery time from the large pulse of CO2 that caused the PETM hyperthermal event. ”
“a layer of organic mud quickly seals the ocean floor”
“from 0.3–5 cm/1000 yr” is not exactly quick.
angech, the abyssal ocean has very little carbonate and thus cannot explain the rise in pH in the deep ocean as you assert. I asked you for evidence to the contrary and you have provided precisely none, just assertion. So where is the evidence?
“Does not alter the fact that ” There are bucket loads of carbonate in the earth’s crust, every subsurface volcanic eruption exposes more to the ocean.”
There isn’t “bucketloads” of carbonates in the basalt erupted from mid ocean ridges, so it is very much the point. The carbonates that do exist on the sea floor are at shallower depths where CaCO3 is supersaturated, and sediments can form, as I and Marco have pointed out repeatedly.
“You agreed to the CO2 release from heating and disruption of the carbonates in the earth’s crust by volcanic events on the seafloor in this comment,”
No, I didn’t because what follows is BBDs statement, not mine. Volcanoes release CO2, that does not mean they release CaCO3, so where is your evidence? Note also that the Carbonates involved are not necessarily from the volcanoes, looking into this I found that apparently a lot of the carbonates are formed by ocean water seeping into cracks in the rock, where the increased temperature subsequently causes the carbonates to precipitate out.
““a layer of organic mud quickly seals the ocean floor”
“from 0.3–5 cm/1000 yr” is not exactly quick”
evasion, the point is the layer is already there, so once the CaCO3 (in the mid-depth ocean, not the abyssal plain) to the depth that experiences bioturbation is used up, there is no more. Below that layer in the mud, any CaCO3 is isolated from the ocean and hence there isn’t an unlimited amount of CaCO3 available as you claim. Current thought is (as IIRC Archer points out) that the fossil fuel emissions are likely to exhaust the CaCO3 currently available, and full removal will require replenishment of the Ca ions from new sediments and chemical weathering on land (via rivers).
I note that angech is disingenuously avoiding the following points:
(i) nobody expects there to be a rapid change in pH of the deep ocean (i.e. below the thermocline)
(ii) “dangerous” (to marine ecosystems) ocean acidification is largely in the surface waters where most ecosystems exist (e.g. coral reefs) and hence a lack of change in pH in the deep ocean is not evidence that there will be no “dangerous” ocean adcidification.
(iii) It is not so much the pH that is the issue, but the change in pH and (as BBD notes) the rate of change of pH, which means that marine ecosystems may be unable to evolve to deal with the environment in which they find themselves.
“from 0.3–5 cm/1000 yr” is not exactly quick”
Just to demonstrate the sophistry involved in angech’s dismissal of the organic clay cap on the seafloor: The Pacific ocean ridge is one with a relatively high spreading rate, generating 80–120 mm/yr of fresh sea floor. The depth at which bioturbation of the clay permits exchange with the ocean is about 8cm. So taking the slowest rate of clay formation as 0.3cm per year (i.e. being as generous to angech as I can by taking the lower bound from his figures), it takes about 27,000 years for the build up of clay to bury newly formed ocean crust, which corresponds to a strip on either side of the ridge (assuming the fastest spreading rate of 120mm/year) 3.24km wide (i.e. a total of 6.48km). Say the ridge extends all the way from the Bering Sea to the Southern ocean, a distance of about 15,500km, then this is an area of approx 100,000 square km. The surface area of the Pacific ocean is about 165.2 million square km, so the area of relatively uncapped newly formed seabed is 0.06% of the ocean sea bed, i.e. a totally negligable amount, and so the effect certainly can’t explain the rise in pH in the abyssal ocean in the transect shown in the diagram under discussion.
I’ll leave it as an exercise for the reader to determine the percentage for a slower spreading ridge (such as the north Atlantic ridge’s approx 25mm/yr) and the upper bound on the rate of formation of the clay.
Of course angech could have performed this “back of the envelope” sanity-check calculation just to see if the rate of formation of the clay is a factor that casts doubt on his argument, but of course he didn’t do that because he is just bullshitting and doesn’t care whether the objection to his argument is valid or not.
And even then, angech still has provided no evidence at all that the erupted magma is rich in CaCO3 anyway!
Dikran , “the abyssal ocean has very little carbonate and thus cannot explain the rise in pH in the deep ocean as you assert”
So what other substance do you postulate to cause the rise in pH.
What substance is causing the rise in pH at the surface?
It cannot be CO2 as this is acidic.
how much calcium carbonate is required to give a pH of 8.1 average for all of the oceans on earth. A massive amount.
CaCO3 is 4% of the overall crust but possibly 8-12% of the surface crust as the sedimentary rocks cover the deeper crust basalts and feldspars. Sedimentary rocks breakdown and are washed into the ocean.
The ocean floor consists of a high percentage of carbonate containing rocks. They exude CaCO3.
I wait for your explanation of the alkaline sea by CO2 outgassing which can only cause acidity.
What mechanism do you suggest?
“So what other substance do you postulate to cause the rise in pH.”
angech, you first need to explicitly admit that you have no evidence to support your assertion. When you have done that, then we might try and discuss the chemistry/physics.
This is especially true if you continue to raise points that have already been addressed, e.g.
“CaCO3 is 4% of the overall crust but possibly 8-12% of the surface crust as the sedimentary rocks cover the deeper crust basalts and feldspars.”
Again you provide no evidence for this whatsoever. The crust basalt in the deep ocean (i.e. below the CCD) is not going to be covered by sedimentary carbonates BECAUSE IT IS BELOW THE CCD! Sorry to shout, but this has been pointed out to you by Marco and myself repeatedly, and you are still ignoring it.
“The ocean floor consists of a high percentage of carbonate containing rocks. They exude CaCO3.”
again you make the assertion, but provide no evidence that abyssal ocean floor is rich in carbonates.
oops, sorry, obviously didn’t close the tags properly 😦
Lets try this again, one question at a time:
Angech, do you agree that the rates of crust production and sediment formation are slow enough that only a small fraction (of the order of 0.1%) of the ocean floor is covered in clay, which keeps greatly impedes an exchange of CaCO3 between the ocean waters and the sea bed below? Yes or no? If no, please point out the error in the “back of the envelope” calculation given above.
doh, obviously should be “of the ocean floor is not covered in clay,”
dikranmarsupial
Lets try this again, one question at a time:
Angech, do you agree that the rates of crust production and sediment formation are slow enough that only a small fraction (of the order of 0.1%) of the ocean floor is not covered in clay?
–
Pamela Hallock, Department of Marine Science, University
of South Florida, 140 7th Avenue South, St. Petersburg, FL 33701n
“Deep-sea sediments, those found at depths greater than about 500 m, cover roughly two-thirds of the Earth. Not surprisingly, there are many kinds of deep-sea sediments. Fortunately, for someone learning about them, the predominant deep sediment is carbonate ooze, which covers nearly half the ocean floor”
–
This is helpful to the discussion.
To be fair I have done a lot of looking for arguments both for and against my contention and the area seems to be as sparsely covered as the abyssal ocean and as impenetrable as the mud/clay on the bottom. If I find any arguments against that make sense I will also post them.
Angech you have avoided the question again.
The question is whether the ooze isolates the bedrock below, a point which you have completely failed to address or acknowledge. If you read either the paper by Archer or the one by Broeker that I gave the URL for, you will know that is the issue. The oceans ability to neutralise the CO2 is in the depth of that ooze that is subject to bioturbation (about 8cm), and that capacity is not unlimited as you asserted, and is expected to be exhausted by out emissions. That directly contradicts your position and you are unwilling to accept it.
Also the mud in the abyssal ocean has no carbonates because it is below the CCD, again you have refused to accept that (it is only the shallower ooze that has carbonate). So that can’t explain the change in pH in the abyssal ocean, which again you have refused to acknowledge.
The carbonates in the ooze is largely from biological sources, not volcanic. Again this is something you have refused to accept and have provided no evidence whatsoever to the contrary.
You have not done a lot of looking for arguments contrary to your contention. I have provided on that directly refutes it (Broeker) and you have obviously not read it.
Just to demonstrate angech’s sophistry, this is what I asked
“Angech, do you agree that the rates of crust production and sediment formation are slow enough that only a small fraction (of the order of 0.1%) of the ocean floor is covered in clay, which keeps greatly impedes an exchange of CaCO3 between the ocean waters and the sea bed below? Yes or no”
the bit in bold is the bit that angech didn’t want to answer because he knows it refutes his contention that the ocean has unlimited CaCO3 to neutralise acidification. This is intellectually dishonest on his part, and I suspect he knows it.
So come on angech, answer the whole question, yes or no?
dikranmarsupial says:
“Angech, do you agree that the rates of crust production and sediment formation are slow enough that only a small fraction (of the order of 0.1%) of the ocean floor is covered in clay, which greatly impedes an exchange of CaCO3 between the ocean waters and the sea bed below? Yes or no”
If only a small fraction of the ocean floor < 0.1% is covered in clay it could not greatly impede the exchange of CaCO3.
I think your question is re a larger amount of clay and whether it is impervious enough to greatly retard CaC03 transfer. This would depend on the type of clay involved and its permeability. Some types might.
In regard to the CCD you have raised some interesting points though I feel it is wrong to say the mud in the abyssal ocean has no carbonates because it is below the CCD,
The mud may have pellets of CaCO3 in it stuck on the surface, The rates of dissolution do depend on the size of the pellets and rate of descent, which can be fast. Also underground rivers can carry vast amounts of CaCO3 silt down.
Have you any thoughts on why so much Calcium carbonate is present throughout the sea and where it came from why when if it was used for shell making it has not become removed from the sea.
dikranmarsupial
“Also the mud in the abyssal ocean has no carbonates because it is below the CCD,”
–
Mud in the abyssal ocean with no carbonates.
because it is below the CCD.
–
Chemistry and knowledge and assertion.
“Mollusks from many different groups live in the deep sea. Our shell-makers can be found at all depth levels of the ocean bottom; no limit is known on the depths at which they can live. Mollusks have been found in the deepest point of all oceans, the Challenger Deep in the Marianas Trench, at 11,022 m (about 36,000 feet) depth”
I presume the 11,022 is well below the CCD.
I presume the molluscs have shells.
Therefore your contention that the CCD is the last word on the chemistry is wrong.
I point out to you the white cliffs of Dover Possibly thousands of meters deep CaCO3.
I point out sea shells in the highest Himalayas just to remind both of us of the vast, interminable aeons that the earths crust has been forming and deforming.
The earths crust, even under the sea, is not just some miserable thin layer deposited in the last 10,000 years.
It is a vast amalgam of CaCo3 deposited over 2 billion years, crushed, serpentined, vapourised frozen, glaciated, vented, heated and pressured into all sorts of minerals and deposits.
It is intermixed with less important stuff for our argument like basalt etc which occur in bigger percentages at greater depths.
The sea sits on the crust. It has input from the earth’s crust not just from the bottom but from every drib and drab where water comes into contact with land as it drains back into the oceans. Wind blows dust particles, some of them CaCo3 into the sea. The volcanic ocean floor rips up this buried crust and periodically exposes great swathes of billion year old CaCO3 to the ravages of the abyssal deep waters [despite the mud layer].
Vast undersea rivers carry silt and debris down to the abyssal depths daily. Bacteria and worms live in the silt and mud, yes even at those depths and burrow and break their way through it exposing rich veins of CaCO3 to the water.
It might look quiet in a nautilus for a week but over a decade the floor is a vibrant freeway of activity, not a quilted protective blanket of CaCO3 less mud.
–
The salient points surely are the supersaturated CaCO3 and pH of 8.1 of the whole ocean overall. How did it get that way?
The Ca part of the mix did not form from the CO2\H2O acid pathway.
It is there because the ocean sits in and on a pitcher of earth which has a CaCo3 matrix which has formed over billions of years,
The ocean is alkaline because of the dissolved earth chemicals in it and available to it at this particular temperature, earth size and water volume. They cannot dissolve out and leave us with pure water which would be acidic with the level of CO2 in the air.
The dissolved salts and CaCO3 are innumerably more abundant and available in the earths crust than all the CO2 that nature and Humanity can produce. The balance is robust, not delicate and is much more a feature of how much CO2 [DIC] is present in water due to the CaCO3 putting it into the atmosphere or stopping it from being absorbed into the sea than a simple current small oversupply.
I am not arguing AGW, or being obstreperous, I am trying to understand the pH conundrum better.
Some of these ideas must make sense
Willard sorry for waxing lyrical!
O.K. angech, there was an obvious typo in the original question, and pointed that out, but forgot to change it when I quoted myself. What I should have asked was:
“Angech, do you agree that the rates of crust production and sediment formation are slow enough that only a small fraction (of the order of 0.1%) of the ocean floor is NOT covered in clay, which keeps greatly impedes an exchange of CaCO3 between the ocean waters and the sea bed below? Yes or no”
As I had already given the calculation, angech ought to have the sense to realise that there was a mistake in the wording (even if I hadn’t pointed it out) and given a straight answer, but he didn’t.
O.K. angech, so lets have a yes or no to the (correctly posed) question.
Just an example of the disingenuous nature of angegh’s Gish gallop, his original claim was:
“There are bucket loads of carbonate in the earth’s crust, every subsurface volcanic eruption exposes more to the ocean. The ocean is alkaline not because of current biological deposition but from leaching from the earth over billions of years”
Note the neutralising carbonate is from below the abyssal mud, not above, but he now argues
“In regard to the CCD you have raised some interesting points though I feel it is wrong to say the mud in the abyssal ocean has no carbonates because it is below the CCD,
The mud may have pellets of CaCO3 in it stuck on the surface, The rates of dissolution do depend on the size of the pellets and rate of descent, which can be fast.”
which of course is completely irrelevant to the question of whether the mud isolates the carbonate containing crust (not that angech has established that the basalt sea floor is particularly rich in carbonate, I suspect it isn’t) from the oceans! Angech is being pedantic here, what I should have said was that there is negligible carbonates in the abyssal mud as it is below the CCD.
so enough of the Gish gallops angech, give a straight answer to my question, and we can see where to go from there. If you make it clear that you are doing your best to evade finding anything we can agree on, then you make it clear that you are just trolling.
Actually, I have just noticed that angech also wrote
“The ocean is alkaline from necessity, it determines most of the CO2 level in the air, not the other way round precisely because of ATTP’ s formulae.”
which is very obviously wrong. The rate at which atmospheric CO2 is rising is slower than the rate at which we are emitting it, which proves beyond reasonable doubt that the natural environment is a net carbon sink and hence cannot be the cause of rising atmospheric CO2. Perhaps angech ought to defend that assertion (as he is basically saying that everybody who researches the carbon cycle is fundamentally wrong on the most basic level, and that he knows better)?
“presume the 11,022 is well below the CCD.
I presume the molluscs have shells.”
Sigh. These molluscs have a very thick periostracum to protect against dissolution of calciumcarbonate. All shell-formers use energy to build up this calciumcarbonate, so when they die, their shell does not last long.
“I point out to you the white cliffs of Dover Possibly thousands of meters deep CaCO3.”
Errr….no, not even close.
“The salient points surely are the supersaturated CaCO3 and pH of 8.1 of the whole ocean overall. ”
Only the surface layer is pH 8.1. The rest is lower in pH, at least in part because in the surface layer we have a higher temperature and therefore less CO2 dissolved, and in part because there are a lot of organisms there that transform CO2 into carbonate. When you go deeper, the process reverses, and you thus get production of CO2 due to decomposition of the organisms that stored the carbonate (hence the drop in pH, along with higher T). Go even lower, and also this process becomes less and less (fewer and fewer organisms), explaining the slight increase in pH towards the abyssal ocean.
Maybe this helps you a little bit further in your understanding:
https://www.nap.edu/read/12904/chapter/4
To be fair, there is some truth in this. As I understand it, the Revelle factor is somewhat set by the alkalinity and hence this does constrain how much of our emissions can be taken up by the ocean. If the pH were much lower, then the Revelle factor would be smaller and a fractional change in atmospheric CO2 would be associated with a larger fractional change in Dissolved Inorganic Carbon. However, this does not change that the reason why atmospheric CO2 is rising, is because of our emissions.
““I point out to you the white cliffs of Dover Possibly thousands of meters deep CaCO3.”
Did the white cliffs of Dover form in deep water? No. Did the white cliffs of Dover form as the result of volcanic eruptions? No. Are the white Cliffs of Dover relevant to the question of abyssal CaCO3? No. Did Angech do his homework? No, he was almost an order of magnitude out. Was this just part of a Gish Gallop to avoid the fact that almost all of ocean crust is isolated from the ocean by mud (and hence any CaCO3 the crust may contain, and angech has not established that oceanic crust is rich in CaC03, can’t greatly affect abyssal pH)? Yes, quite probably.
Thanks for the explanation and the link Marco!
ATTP I agree.
I should add though that it doesn’t “determine[s] most of the CO2 level in the air” on the sort of timescales we are discussing (less than a few centuries), currently we do.
Indeed; it’s really some fraction of our emissions that will remain in the atmosphere.
dikranmarsupial says:
“I point out to you the white cliffs of Dover Possibly thousands of meters deep CaCO3.”
Did the white cliffs of Dover form in deep water ? No.
I did not say they formed in abyssal water. They could form in reasonably deep water from calciferous ooze. My point was they are one of numerous forms of CaCO3 in the earths crust over 2 billion years.
Did the white cliffs of Dover form as the result of volcanic eruptions? No.
They were actually uplifted by volcanic activity to be where they are
Are the white Cliffs of Dover relevant to the question of abyssal CaCO3? No.
I was using them as one of a number of examples to show how long CaCO3 has been forming for and in how many ways it has formed to be such an abundant component of the earths crust.
If you refuse to see the relevance of the abyssal oceans sit on the earth’s crust [which has multiple forms of CaCO3 in it] so be it.
A book on Oceanography Invitation to Marine science that I cannot copy from has chapters headed
7.1 water is a powerful solvent
7.2 Seawater consists of water and dissolved solids
The components of ocean salinity came from and have been modified by earth’s crust
The ratio of dissolved solids in the earths crust is constant.”
This last point is most telling for the argument I have been putting across,
the previous ones confirm my position.
Did Angech do his homework?
he was almost an order of magnitude out.
You are correct there, I thought they could have gone a lot deeper.
Apologies.
Was this just part of a Gish Gallop to avoid the fact that almost all of ocean crust is isolated from the ocean by mud (and hence any CaCO3 the crust may contain, and angech has not established that oceanic crust is rich in CaC03, can’t greatly affect abyssal pH)?
Sorry this is not correct in so many ways.
Mud covers only 38%. Sediment can be up to a kilometer thick It has life in it and it is beinf acted upon by rivers earthquakes volcanoes and has incoming debris all the time and only some type of clay are impermeable.
angech wrote “They [the white cliffs of dover] could form in reasonably deep water from calciferous ooze. My point was they are one of numerous forms of CaCO3 in the earths crust over 2 billion years”
O.K. so angech immediately doubles down. The white cliffs of Dover did not form in reasonably deep water. How deep do you think the English channel is? Hint there was a land bridge across it a few tens of thousands of years ago. Given that we were talking about the alkalinity of the deep ocean, the white cliffs of Dover are a complete red herring. It is a shame you can’t admit that an double down instead.
“They were actually uplifted by volcanic activity to be where they are”
more evasion, the relevance of volcanic activity is the question of whether the basalt ocean crust has CaCO3 content. If the white cliffs of Dover were uplifted by volcanic processes (for which you provide no evidence) then that still doesn’t mean that the ocean floor has CaCO3.
Did I mention you would be better off avoiding Gish Gallops?
“I was using them as one of a number of examples to show how long CaCO3 has been forming for and in how many ways it has formed to be such an abundant component of the earths crust.”|
Again evasion, the sediments forming the WCoD formed in shallow water and hence do not imply that oceanic crust has much CaCO3.
“If you refuse to see the relevance of the abyssal oceans sit on the earth’s crust [which has multiple forms of CaCO3 in it] so be it.|”
now this really is rhetorical bullshit as angech has provided no evidence whatsoever that the crust under the oceans (which is of a very different character to the crust under land or continental shelf) has a lot of CaCO3 in it). I know the continental crust and shallow ocean bed has plenty of CaCO3 in it, but that doesn’t mean the basalt ocean crust does.
angech quotes from a book:
“The components of ocean salinity came from and have been modified by earth’s crust”
yes, this is the chemical weathering mechanism that governs atmospheric CO2 on very long (geological) timescales. The crust involved is the continental crust, which supplies materials eroded into rivers. It does not mean that the oceanic crust is rich in CaCO3.
“Mud covers only 38%. Sediment can be up to a kilometer thick It has life in it and it is beinf acted upon by rivers earthquakes volcanoes and has incoming debris all the time and only some type of clay are impermeable.”
Citation needed for the 38%. I know sediment layers can be very thick, but that doesn’t mean the sediment below the CCD contains a non-negligible amount of CaC03, so again you are using the red herring of shallow ocean sediment to avoid the point that the abyssal ocean has very little. This is just more sophistry. Note also that it is only the top layer of the mud that undergoes bioturbation (about 8cm according to Broeker) that is in exchange with the oceans, so you sediment can be kilometers thick, but it is still sealed from the ocean if it is covered by a foot of mud.
Right, so one last time, what is your evidence that the abyssal ocean crust (formed from eruptions from the mid ocean ridges) has a substantial amount of CaCO3. Another Gish galllop would be an admission that you don’t want to answer this question.
We need an oceanographer.
-Citation needed for the 38%, done
“Main article: Pelagic red clay Pelagic sediment Wiki
Red clay, also known as either brown clay or pelagic clay, accumulates in the deepest and most remote areas of the ocean. It covers 38% of the ocean floor and accumulates more slowly than any other sediment type,”
-that doesn’t mean the sediment below the CCD contains a non-negligible amount of CaC03,
“Containing less than 30% biogenic material, it consists of sediment that remains after the dissolution of both calcareous and siliceous biogenic particles while they settled through the water column.”
Less than 30% should contain significant CaCO3 .
evidence that the abyssal ocean crust (formed from eruptions from the mid ocean ridges) has a substantial amount of CaCO3.
research over the past decade or so shows that they teem with a wide variety of microbial life
Marine Life (CeDAMar) have found an extremely high level of biodiversity on abyssal plains, with up to 2000 species of bacteria, 250 species of protozoans, and 500 species of invertebrates (worms, crustaceans and molluscs)
it is only the top layer of the mud that undergoes bioturbation (about 8cm according to Broeker) that is in exchange with the oceans.
Of course, and it has CaCO3 in it and is constantly replenished by wind borne, pelagic and biological life breakdown in this layer.
“Right, so one last time, what is your evidence that the abyssal ocean crust (formed from eruptions from the mid ocean ridges) has a substantial amount of CaCO3. ”
I would merely point out that you are standing on it, the evidence that is.
The ground your house is built on, the fields your kids played sport on, The mountains you go to ski on were all once part of an abyssal ocean floor.
You can have a basalt outpouring at a mid ocean ridge creating new ocean crust, but as it spreads out it becomes intermixed with the rest of the older crust.
That is what 4 billion years does to ocean crust.
The older crust on land is constantly running back to the sea and covering the new crust.
As the crust spreads it also gets covered by debris from older crust, with CaCO3 in it from biogenic activity.
It has 2 billion years of CaC03 running of the hills and being blown through the air and manufactured in the oceans.
Note the abyssal plains , 3000-6000 meters are rich in life and are not composed purely of basalt and are not at the ocean ridges. They tend to be back a bit closer to the continental shelves.
They are covered by sediment up to a kilometer deep which is what is in contact with the ocean
The ocean itself is supersaturated with CaCO3 so the whole crust is bathed in it anyway.
I know the continental crust and shallow ocean bed has plenty of CaCO3 in it, but that doesn’t mean the basalt ocean crust does.
Pure basalt obviously does not. The problem is that the ocean crust is not pure basalt, The only place in the ocean crust where this is slightly correct is at the ridges where basalt plumes are occurring, and even then they are erupting through a layer of CaCo3 containing material.
“O.K. so angech immediately doubles down. The white cliffs of Dover did not form in reasonably deep water. How deep do you think the English channel is? Hint there was a land bridge across it a few tens of thousands of years ago.”
The English Channel average depth of about 120 m (390 ft) at its widest part, reducing to a depth of about 45 m (148 ft) between Dover and Calais.
–
The white cliffs of Dover never formed in the English Channel.
–
The Chalk is part of the Upper Cretaceous and ranges in ages from about 100 million years to 65 million years. , estimates of the depth vary but deeper than the English Channel. the Chalk of Kent being deposited in a depth range of 100-300m. some marginal (shallow) marine deposits being recorded both in Devon and NW France.The chalk downs were lifted up to form the North Downs because of crumpling of the earth’s crust caused by the slow movement north of the African tectonic plate colliding with the European tectonic plate.
The English Channel is a somewhat new formation. The first 425,000 years ago, an ice-dammed lake in the southern North Sea overflowed and broke the Weald-Artois chalk range in a catastrophic erosion and flood event. a second flood about 225,000 years ago .
–
It is this understanding of the sheer time scale that is important. For all we know there have been many occurrences of far bigger chalk deposits in the past hundreds of millions of years before this, now reformed as marble or limestone nor dissolved back into the sea.
The depth is irrelevant
The cliffs are an example of how CaCO3 gets into the crust all over the earth and hence can be found [not formed] even in abyssal depths
Angech, you might want to read that Wikipedia article on pelagic sediment in more detail. It also has a nice map, with thickness of the sediment. Note the prevailing dark blue color, and read what it means.
I just keep on returning to my earlier observation that you stop reading when you think you encountered something that proves your point, and then ignore all the rest that doesn’t.
O.K. angechs is dishonest from his very first item in yet another Gish gallop, this time laughable selective quoting once again, here is angch’s quote:
-Citation needed for the 38%, done
“Main article: Pelagic red clay Pelagic sediment Wiki
Red clay, also known as either brown clay or pelagic clay, accumulates in the deepest and most remote areas of the ocean. It covers 38% of the ocean floor and accumulates more slowly than any other sediment type,”
Here is what the wikipedia page says:
“Red clay, also known as either brown clay or pelagic clay, accumulates in the deepest and most remote areas of the ocean. It covers 38% of the ocean floor and accumulates more slowly than any other sediment type, at only 0.1–0.5 cm/1000 yr.[1] Containing less than 30% biogenic material, it consists of sediment that remains after the dissolution of both calcareous and siliceous biogenic particles while they settled through the water column.“
So the very next sentence directly contradicts angech’s argument by pointing out that red pelagic mud contains very little calcareous material which had already dissolved in the water column. Sorry angech, again that it intellectually utterly dishonest.
Appologies, I should have read further to see that angech did quote the second sentence, however he responds to it by saying “Less than 30% should contain significant CaCO3 .” no it doesnt’t CaC03 is calcereous material, which the sentence says has already dissolved in the water column, so you still haven’t established the point.
So, what is your evidence that pelagic red mud contains significant CaCO3, not assertions, not Gish gallops, but evidence supported by references.
Maybe angech should read this again.
Just to demonstrate angech’s sophistry, here is a good bit
““O.K. so angech immediately doubles down. The white cliffs of Dover did not form in reasonably deep water. How deep do you think the English channel is? Hint there was a land bridge across it a few tens of thousands of years ago.”
The English Channel average depth of about 120 m (390 ft) at its widest part, reducing to a depth of about 45 m (148 ft) between Dover and Calais.
–
The white cliffs of Dover never formed in the English Channel.”
Note the point was that the White Cliffs of Dover didn’t form in deep water, so of course rather than admit that they didn’t form in deep water, goes of on a bit of irrelevant pedantry. The point being made is that the whole of the south east of england was recently joined by a land bridge to Europe, so where was this deep water for the cliffs to form in? Nowhere.
ATTP there is a similar section in the paper by Broeker that I linked to above and angech ignored that as well.
angech might find this interesting
“The depth at which surface production of CaCO3 equals dissolution is called the calcium carbonate compensation depth (CCD). Above this depth, carbonate oozes can accumulate, below the CCD only terrigenous sediments, oceanic clays, or siliceous oozes can accumulate. The calcium carbonate compensation depth beneath the temperate and tropical Atlantic is approximately 5,000 m deep, while in the Pacific, it is shallower, about 4,200-4,500 m, except beneath the equatorial upwelling zone, where the CCD is about 5,000 m. The CCD in the Indian Ocean is intermediate between the Atlantic and the Pacific. The CCD is relatively shallow in high latitudes.
…
The preservation potential of the various kinds of carbonate shells and skeletons differs. Pteropod shells are aragonite, a less stable form of CaCO3. Pteropod shells dissolve at depths greater than 3,000 m in the Atlantic Ocean and below a few hundred meters in the Pacific. Calcitic planktic foraminiferal tests, especially small tests of juvenile spinose foraminifera, dissolve more readily than coccoliths, which are also made of calcite. Pelagic sediments from relatively shallow depths in low latitudes are often dominated by pteropods shells, at intermediate depths by foraminiferal tests, below the lysocline and above the CCD by coccoliths, and below the CCD by red clays.“
Note the first paragraph quoted explicitly states that carbonate sediments don’t accumulate below the CCD (there may be some carbonate in the top layers of the mud, but it gets dissolved before it can get buried deep enough to be isolated from ocean dissolution). The second paragraph strongly implies that there is very little or no carbonate in red clays.
“Note the first paragraph quoted explicitly states that carbonate sediments don’t accumulate below the CCD (there may be some carbonate in the top layers of the mud, but it gets dissolved before it can get buried deep enough to be isolated from ocean dissolution)”
Wrong
Commonly stated on Wiki. disproved in at least 3 places, all hard to copy.
Scientific Exploration of the South Pacific: Proceedings of a Symposium Held …
edited by Warren Scriver Wooster states there may be 200 meters of calciferous ooze under red clay due to sinking of the basin the ooze had formed in and being covered by red clay before it could dissolve.
Images show pictures of the calciferous ooze extending down to the CCD and under it, try “calcareous ooze found under red clay ocean images”.
Note they did a drilling expedition, samples. virtually all of them, showed material going back 50-120 million years dated due to the shells found in the samples . Where were the fossils?
Underneath the red clay and more importantly, undissolved.
–
“The second paragraph strongly implies that there is very little or no carbonate in red clays.”
Correct.
Furthermore I can see statements to this effect in multiple places.
To deny it would be to go against
Wally Broeker “The phenomenon I observed is that the Earth’s average climate temperature has been increasing for a century due to man-made increases in levels of greenhouse gases, which in turn corresponds to exponentially extreme weather effects; the slight but significant increase in global surface temperature we can predict will result in rising sea levels, melting ice regions, expansion of desert zones, and more. The global effects will be catastrophic and we are approaching the point of no return. Some think we’ve passed that point. The governing scientific board of every developed nation on Earth has declared global warming to be real.”
Had not come across him before but I see where the ideas on C02 retention time in the atmosphere come from now.
Does anyone have a copy of a core sample red clay no CaCO3 present lying around to show me?
Either provide evidence, or stop making these claims.
“Commonly stated on Wiki. disproved in at least 3 places, all hard to copy.”
Bullshit. The CCD is defined as the depth at which the production of carbonates is exceeded by the rate of its dissolution (in the sediments as well as in the water column). You only need basic maths skills to tell you that carbonate sediments cannot accumulate if they are being dissolved faster than they form – duh!
“Warren Scriver Wooster states there may be 200 meters of calciferous ooze under red clay due to sinking of the basin the ooze had formed in and being covered by red clay before it could dissolve.”
and if it is under the red clay it is unavailable to neutralise acid in the oceans, so that hardly makes your point. Also it says that the carbonate in UNDER the red clay, not that the red clay CONTAINS carbonate.
“Underneath the red clay and more importantly, undissolved.”
underneath, not in and hence not available for neutralising acid in the oceans, so again doesn’t support your contention. Note that the carbonate deposits millions of years old did not necessarily form below the CCD, geology changes on geological timescales.
BTW angech demonstrated he doesn’t even know what the CCD is when he wrote
“The ocean itself is supersaturated with CaCO3 so the whole crust is bathed in it anyway.”
The ocean below the CCD is not supersaturated with CaCO3, so the whole crust is definitely NOT bathed in it anyway.
Now for the most spectacular bit of bullshit in the post
I wrote:
“The second paragraph strongly implies that there is very little or no carbonate in red clays.”
angech replies
“Correct. Furthermore I can see statements to this effect in multiple places. To deny it would be to go against Wally Broeker”
However angech earlier wrote:
“-Citation needed for the 38%, done
“Main article: Pelagic red clay Pelagic sediment Wiki
Red clay, also known as either brown clay or pelagic clay, accumulates in the deepest and most remote areas of the ocean. It covers 38% of the ocean floor and accumulates more slowly than any other sediment type,”
-that doesn’t mean the sediment below the CCD contains a non-negligible amount of CaC03,
“Containing less than 30% biogenic material, it consists of sediment that remains after the dissolution of both calcareous and siliceous biogenic particles while they settled through the water column.”
Less than 30% should contain significant CaCO3 .”
Yes, that is right, your eyes aren’t deceiving you, here is angech stating that red clays should contain significant CaCO3, going against Broeker. It could just be that angech was being sarcastic about Broeker, but really if he is going to dismiss the writings of someone who has actually studied the ocean carbon cycle (indeed one of the most eminent researchers in the field), then that makes it clear that this discussion is not actually about the science.
“Had not come across him [Broeker] before”
You really ought to be embarrassed to admit in this way that you had not looked at the source I referenced much earlier in the thread (or indeed made any attempt to look into the science of the oceanic carbon cycle).
Following angech’s advice “Images show pictures of the calciferous ooze extending down to the CCD and under it, try “calcareous ooze found under red clay ocean images”.” I had a look, but none of the images as far as I can see support his position, but this one is great
I wonder why angech didn’t mention that one? LOL
How about this one:
Note the carbonates formed above the CCD, so that doesn’t support his position. As the carbonates are covered by pelagic red clay they are isolated from the ocean, so they can’t neutralise acid, so that doesn’t support his position either. Indeed the reason the carbonates are not dissolved by the undersaturated abyssal waters is presumably exactly because they are covered in pelagic red mud.
this one states that calcereous oozes are scarce below 5000m (i.e. below the CCD), wonder why angech didn’t mention that one.
calcereous oozes generally confined to ocean shallower than the CCD, again no mention of this from angech
looks to me as if angech was being rather selective in what he reported of the images that his search produced. Again, rather didingenuous.
Oops, the URLs for the first two images shouldn’t have the bits after the ? (which is presumably why they don’t appear).
[Mod: fixed]
dikranmarsupial says: November 21, 2016 at 9:26 am
“The ocean below the CCD is not supersaturated with CaCO3, so the whole crust is definitely NOT bathed in it anyway.”
Care to address this comment, as in retract it, as it is wrong.
I see now I have been drawn into a strawman argument, which I do not mind.
The distraction is the red clay to take away from my point that the water rests on the earth and that the earth puts the CaCO3 into the water.
Arguments about how the CaCO3 gets into the water from the earth are concentrated on one small part of the mechanism where it is harder to occur, not on the argument itself.
Well done.
Oh, BTW, thanks for putting up the second and third pictures. Your computer skills are way above mine and they are very helpful. The second one shows the calcareous ooze right on top of the mid ocean ridges and further out under the red clay.
–
Confirming my point “There are bucket loads of carbonate in the earth’s crust, every subsurface volcanic eruption exposes more to the ocean” despite your assertion to the contrary.
Dikran Marsupial says: November 9, 2016 at 9:56 am “. I don’t think this is necessarily correct.”
–
You also said.
“the reason that land based volcanoes give off a lot of CO2 is because they are largely erupting magma formed from subducted crust from shallower oceans that has carbonate sediments.”
–
The magma is the same, molten basalt under the sea and on land.
Any component of “subducted crust from shallower oceans that has carbonate sediments” can only be present in the ocean ridge volcanoes under the sea. That is where the subduction takes place.Subducted crust is melted,gone, not crust no more.
Land based volcanoes give off extra CO2 because the magma passes through ordinary crust with layers of chalk limestone marble etc, carbonate sediments from 2 billion years of non subducted crust formation of sediments from shallower oceans.
–
Finally, you state
“this one states that calcareous oozes are scarce below 5000m (i.e. below the CCD),”
the statement should read but doesn’t “recent calcareous oozes” as in the next sentence which you may have missed it says “ancient calcareous oozes at greater depths if moved by sea floor spreading”.
By the picture accompanying these calcareous oozes are abundant not scarce you would agree.
The term scarce applies to CaCO3 deposits on the red clay surface below the CCD, One wonders why they did not say non existent?
Let us agree the pot is a stew of CaCO3 and that the CO2 levels run of this normally.
O.K. angech is bullshitting straightaway:
“dikranmarsupial says: November 21, 2016 at 9:26 am
“The ocean below the CCD is not supersaturated with CaCO3, so the whole crust is definitely NOT bathed in it anyway.”
Care to address this comment, as in retract it, as it is wrong.
I see now I have been drawn into a strawman argument, which I do not mind.”
It was angech that asserted that
“The ocean itself is supersaturated with CaCO3 so the whole crust is bathed in it anyway.”
so how can he be drawn into a strawman argument by me fact checking HIS assertions. Sorry angech but you really are just bullshitting, and I suspect you know it.
@The distraction is the red clay to take away from my point that the water rests on the earth and that the earth puts the CaCO3 into the water.”
The red clay isn’t a distraction, the red clay separates the ocean from the crust and prevents an exchange of CaCO3, so regardless of how much CaCO3 is in the crust, it is not available to neutralise acid in the ocean, as pointed out by Archer, as pointed out by Broeker, as pointed out my me and others. That means that the abyssal ocean below the CCD does not have unlimited CaCO3.
“By the picture accompanying these calcareous oozes are abundant not scarce you would agree.”
No, they are scarce below the CCD, which is the point being made, which you are evading.
And just to add, calcereous oozes are abundant above the CCD, but once the CaCO3 they contain is used up to neutralise acid in the oceans, the top layer becomes a non-calcereous ooze, which then isolates the calcereous ooze below from exchange with the oceans, and the neutralisation stops at that point until the CaC03 is replenished from chemical weathering, so there isn’t an unlimited supply of CaCO3 above the CCD either, as pointed out by Broeker.
“The ocean below the CCD is not supersaturated with CaCO3, so the whole crust is definitely NOT bathed in it anyway.”
Care to address this comment, as in retract it, as it is wrong.”
no, because it isn’t wrong.
Degree of saturation of CaCO3 in the oceans
Yuan-Hui Li,Taro Takahashi & Wallace S. Broecker
DOI: 10.1029/JC074i023p05507
Abstract
The degree of saturation of CaCO2 in the Pacific and Atlantic oceans has been calculated from measurements of the partial pressure of CO2 gas and of the total content of dissolved inorganic CO2. Lyman’s apparent dissociation constants for carbonic and boric acid at 1 bar, MacIntyre’s apparent solubility product of CaCO3 in sea water at 1 bar, and the Disteches’ determinations of the pressure dependence of these parameters were used for the calculation. The results indicate that the crossover from supersaturation to undersaturation for CaCO3 occurs at a water depth between 500 and 3000 meters for calcite and at about 300 meters for aragonite in the Pacifiic, and between 4000 and 5000 meters for calcite and between 1000 and 2500 meters for aragonite in the North Atlantic Ocean. This difference between the Pacific and Atlantic oceans may be attributed to a difference in the amount of CO2 dissolved in these waters. Such a difference may, in turn, be caused by a difference in the residence times in these waters. The marked decrease of CaCO3 content in the ocean bottom sediments observed at water depths greater than 3500 meters in the Pacific and 4500 meters in the Atlantic appears to reflect a transition of the sea water from saturation to undersaturation with respect to calcite.
So angech, do you agree that the ocean below the CCD is not supersaturated with CaCO3, yes or no?
dikranmarsupial
“So angech, do you agree that the ocean below the CCD is not supersaturated with CaCO3, yes or no?”
Full comment please.
“The ocean below the CCD is not supersaturated with CaCO3, so the whole crust is definitely NOT bathed in it anyway.”
Italics “NOT”. Your words.
The comment, in full, is wrong because the ocean contains lots of CaCO3 at all levels, more so where the pH is higher e.g at the surface due to evaporation [supersaturated] and at depth due to increasing pressure and low temperature.
Apropos the comment the whole crust below the CCD is bathed in lots of CaCO3 because the ocean water below the CCD has lots of CaCO3 in it
The crust therefore is bathed in it.
–
“And just to add, calcareous oozes are abundant above the CCD, but once the CaCO3 they contain is used up to neutralize acid in the oceans, the top layer becomes a non-calcareous ooze”
The calcareous oozes do not get used up, they accumulate and grow. That is the reason or being a Calcareous ooze in the first place. Nice try.
The ocean is alkaline so they do not have to exert themselves very much.
Can we stop now, please.
angech what part of
“The results indicate that the crossover from supersaturation to undersaturation for CaCO3 occurs at a water depth between 500 and 3000 meters for calcite and at about 300 meters for aragonite in the Pacifiic, and between 4000 and 5000 meters for calcite and between 1000 and 2500 meters for aragonite in the North Atlantic Ocean.”]
do you not understand? The ocean below the CCD (4200 – 4500m for the Pacific, about 5,000m for the Atlantic.) is not saturated with CaC03, or are you saying that Broeker is wrong?
As to Apropos the comment the whole crust below the CCD is bathed in lots of CaCO3 because the ocean water below the CCD has lots of CaCO3 in it
The crust therefore is bathed in it.”
the waters below the CCD are undersaturated with CaCO3, so any CaC03 in contact with those waters will be dissolved by it and hence it will not accumulate there (unless buried by pelagic red clay, in which case it isn’t available for neutralising acid). It would be more accurate to say that the sea floor below the CCD is bathed in water which dissolves any CaCO3 that reaches it.
“The calcareous oozes do not get used up, they accumulate and grow. ”
not if the CaC03 is used up to neutralise acidity in the oceans due to anthropogenic CO2 emissions.
angech,
Given that you continue to assert things without providing any context, or evidence, this would probably be best.
From: 6.19 – The Oceanic CaCO3 Cycle, W.S. Broecker (doi:10.1016/B0-08-043751-6/06119-3):
6.19.13. Neutralization of Fossil Fuel CO2
The ultimate fate of much of the CO2 released to the atmosphere through the burning of coal, oil, and natural gas will be to react with the CaCO3 stored in marine sediments (Broecker and Takahashi, 1977; Sundquist, 1990; Archer et al., 1997). The amount of CaCO3 available for dissolution at any given place on the seafloor depends on the calcite content in the sediment and the depth to which sediments are stirred by organisms. The former is now well mapped and the latter has been documented in many places by radiocarbon measurements. The amount of CaCO3 available for dissolution at any given site is given by
where h is the depth of bioturbation, ρ is the water-free sediment density, and fc is the weight-fraction calcite. The high-CaCO3 sediments that drape the oceans’ ridges and plateaus typically have ∼90% CaCO3 and a water-free density of 1 g cm−3. The bioturbation depth in these sediments averages 8 cm. Hence, the upper limit on amount of CaCO3 available for dissolution in such a sediment is 72 g cm−2. As roughly one quarter of the seafloor is covered with calcite-rich sediments, this corresponds to ∼6.3×1019 g CaCO3 (i.e., 7,560 Gt C). This amount could neutralize 6.3×1017 mol of fossil fuel CO2. This amount exceeds the combined oceanic inventory of dissolved CO32− (1.6×1017 mol) and of dissolved VHBO3− (0.8×1017 mol). It is comparable to the amount of recoverable fossil fuel carbon.
I say ‘upper limit’ because once this amount of CaCO3 has been dissolved, the upper 8 cm of the sediment would consist entirely of a noncarbonate residue. As molecular diffusion through such a thick residue would be extremely slow, the rate of dissolution of CaCO3 stored beneath this CaCO3-free cap would be minuscule, and further neutralization would be confined to the fall to the seafloor of newly formed CaCO3.
The rate of this dissolution of the CaCO3 stored in the uppermost sediment will depend not only on the magnitude of the reduction of the deep ocean’s CO32−content, but also on the rate at which the insoluble residue is stirred into the sediment. This bioturbation not only homogenizes the mixed layer, but is also exhumes CaCO3 from beneath the mixed layer.
——————————————————————————————————–
Now this makes it pretty clear that the only CaCO3 available for neutralising anthropogenic CO2 emissions is that in the top (biopurterbed – about 8cm) layer of the ocean sediment, and as the paragraph in bold states, once that is used up, the top (now carbonate free) layer isolates the sediments and crust below from the ocean and hence that is where neutralisation stops until fresh CaC03 becomes available. This pretty much says the same thing as the quote from Archer that ATTP gave above. I’ll repeat it here:
“The sediment response is composed of two components: a fast pH-neutralizing reaction of CO2 with CaCO3 on the sea floor (a process called chemical erosion), followed by a longer timescale reaction of CO2 with carbonates on land (weathering) (Archer et al. 1997, Ridgwell&Hargreaves 2007). The amount of CaCO3 on the sea floor available to dissolve is limited by the formation of a clay layer on the sea floor, the nonreactive material remaining after CaCO3 dissolves, which impedes further CaCO3 dissolution (Broecker & Takahashi 1978, Archer et al. 1997). The 5000 Pg C CO2 release is close to enough to deplete the available CaCO3 stock of the ocean, leaving excess weathering over CaCO3 burial to drive the CO2 neutralization.”
Sorry angech, you are simply wrong, there is not unlimited CaCo3 in the ocean crust available to neutralise acid from anthropogenic CO2 emissions. All that is available is in the to 8-10cm of sediments, and sediments below the CCD have very little CaCo3 to begin with.
So angech, are both Archer and Boreker wrong about this?
“are both Archer and Broecker wrong about this?”
“there is not unlimited CaCo3 in the ocean crust available to neutralize acid from anthropogenic CO2 emissions. All that is available is in the to 8-10cm of sediments, and sediments below the CCD have very little CaCo3 to begin with.”
Archer answers this,
“The sediment response is composed of two components: a fast pH-neutralizing reaction of CO2 with CaCO3 on the sea floor (a process called chemical erosion), followed by a longer timescale reaction of CO2 with carbonates on land (weathering) (Archer et al. 1997″
Sediment does not have to be below the CCD to be available to neutralize the CO2.
As Archer says carbonate sediment also exists on land.
I presume he is educated enough [it is his field] to understand about aquifers and subterranean water flows and CaCO3 being under the land surface which means he is being disingenuous in saying the process is on a longer time scale on land or has not bothered to factor in the degree of neutralization available and return of soluble CaCO3 to the sea.
–
“roughly one quarter of the seafloor is covered with calcite-rich sediments, this corresponds to ∼6.3×1019 g CaCO3 (i.e., 7,560 Gt C). This amount could neutralize 6.3×1017 mol of fossil fuel CO2.”
What he is saying is ATTP’s formula in reverse.
From the alkalinity and pH of the ocean we can deduce how much CaCO3 is available in the earth’s crust to keep the pH at 8.2.
He has obviously also forgotten to take the land circulatory component of the ocean, ie the humidity, rain rivers lakes aquifers and underground water into account but there is an easy way to do this which I have pointed out ad infinitum
Reducto ad absurdum If the pH in the ocean to the CaCO3 in the earths crust is a given, then the amount of carbonate freely available to keep the water at this overall pH is equivalent to the amount of freely available CaCO3 to neutralize excess CO2.
This CaCO3 already exists on the available sea floor and in the various land sources.
As for the CaCO3 underneath the red clay being unavailable there is no reason for deeper aquifers of water to run through it and bring it back to the ocean [Kublai Khan, Alph’s sacred river ran through canyons unfathomable to man]
Nor for it to be in association with water anyway in which case water always [like love] finds a way.
“I presume he is educated enough [it is his field] to understand about aquifers and subterranean water flows and CaCO3 being under the land surface which means he is being disingenuous in saying the process is on a longer time scale on land or has not bothered to factor in the degree of neutralization available and return of soluble CaCO3 to the sea.”
Gee, oh exalted all-knowledgeable Angech, please tell us how much CaCO3 aquifers and subterranean water flows return to the ocean and how this compares to weathering. Come on, then, you seem to know so much that you even see through Archer’s game, or so you claim, so you will be able to tell us the answer right away. Right?
Disingenuous argument from angech yet again. I wrote:
“there is not unlimited CaCo3 in the ocean crust available to neutralize acid from anthropogenic CO2 emissions. All that is available is in the to 8-10cm of sediments, and sediments below the CCD have very little CaCo3 to begin with.”
angech replies:
“Archer answers this, “The sediment response is composed of two components: a fast pH-neutralizing reaction of CO2 with CaCO3 on the sea floor (a process called chemical erosion), followed by a longer timescale reaction of CO2 with carbonates on land (weathering) (Archer et al. 1997″
That in no way answers my point, because the chemical erosion that Archer mentions is limited to the top 8-10 cmy of the sediments, for the reasons I have already explained, and the chemical weathering component takes tens to hundreds of thousands of years.
Sediment does not have to be below the CCD to be available to neutralize the CO2.
More disingenuous argument from angech, I never said it did. I mentioned the lack of calcereous ooze below the CCD to show that your argument that the crust is bathed in CaCO3 is incorrect.
which means he is being disingenuous in saying the process is on a longer time scale on land or has not bothered to factor in the degree of neutralization available and return of soluble CaCO3 to the sea.
The hubris of this is hilarious, Archer and Broeker have spent their entire careers working on this stuff (the CaCO3 cycle in particular is one of Broekers main research interests) and yet angech thinks he knows better (but yet again can supply no actual evidence to back him up).
angech writes:
“roughly one quarter of the seafloor is covered with calcite-rich sediments, this corresponds to ∼6.3×1019 g CaCO3 (i.e., 7,560 Gt C). This amount could neutralize 6.3×1017 mol of fossil fuel CO2.”
What he is saying is ATTP’s formula in reverse.
You will note that angech ignores the paragraph that follows immediately afterwards, that iI put in bold just to highlight the fact that this was the important bit:
“I say ‘upper limit’ because once this amount of CaCO3 has been dissolved, the upper 8 cm of the sediment would consist entirely of a noncarbonate residue. As molecular diffusion through such a thick residue would be extremely slow, the rate of dissolution of CaCO3 stored beneath this CaCO3-free cap would be minuscule, and further neutralization would be confined to the fall to the seafloor of newly formed CaCO3.”
It is deeply shabby that angech can’t admit this.
“He has obviously also forgotten to take the land circulatory component of the ocean, ie the humidity, rain rivers lakes aquifers and underground water into account but there is an easy way to do this which I have pointed out ad infinitum”
So angech hasn’t bothered to read the paper yet. Of course Broeker is aware of the rate at which carbonates enter the ocean from chemical weathering. Angech provides no evidence whatsoever that the amount entering from aquifers and underground water is non-negligible, yet he thinks he knows better than Broeker, which again is laughable hubris.
“As for the CaCO3 underneath the red clay being unavailable there is no reason for deeper aquifers of water to run through it and bring it back to the ocean [Kublai Khan, Alph’s sacred river ran through canyons unfathomable to man]
Nor for it to be in association with water anyway in which case water always [like love] finds a way.
Poetry is not a form of scientific evidence, and I think this paragraph is about the lamest attempt to avoid accepting what a scientist says that I have ever seen (and I have been reading climate blogs for a fair while).
Thank you for putting your side Dikran, and Marco
not my side angech, Archer and Broekers’ side, and you have provided no evidence that they are wrong, just assertion. It is a shame you can’t admit that you were wrong and that the only CaCO3 available is the top 8-10 cm of the sediment that is “bioperturbed”, being wrong happens to us all, and it is nothing to be ashamed of, … not being able to admit that you are wrong, rather less so.
All the stubborn denialism in the world has zero effect on actual ocean chemistry, angech. You seem to have forgotten that nature refutes you.
Panel (c) *again*:
BBD refutes or confirms depends on which part of actual ocean chemistry you are referring to.
If you wish to confine yourself to saying adding acid to a solution makes the solution more acidic we are both in agreement.
If you wish to discuss the ocean you actually have to specify things and be prepared for logical arguments.
The graphs you use and the time period they are in and the articles you previously linked to them suffer from major flaws.
I maintain that the earth/ ocean/ air complex that currently exists has had an extremely stable existence for 2 billion years and is quite resistant to severe pH change.
You have chosen to conflate pH of the surface ocean with that of the whole ocean quite deliberately in your responses and avoided the issues of the actual amount of pH change and at what depth, position and time and what constitutes severe.
If you are prepared to set the limits then we can have a productive discussion in those limits, otherwise we have red (clay) herrings.
Two simple observations, the graphs show some severe loss of CaCO3 at some of 5 sites only at the PETM event time frame in one ocean only. That such an event happened 55 million years ago is indisputable.
The cause is not known, the amount of CO2 is not known, the length of time is not known as carbon dating is assessed in ten thousand year chunks at that time period and the amount of surface acidity increase (surface only**) is not known and cannot be accurately measured, only poorly guesstimated by models. We only know that some surface acidity would have happened given such an event.
As to the confirmation, Zachos et al 2005. Did you bother to read it?
Two dating shell samples only of Boron changes possibly 16,000 years apart of shells that looked slightly like curent day shells showed changes, that if they reacted the same way as current, 55 million later shell types do might show a decrease in pH. Plus the layer they took the shells from may not have had any shells in (remember they all dissolved? at this time) so the samples, all two, were actually from less effected layers before and after the event and the seperate carbon dating was a bit tricky as well, no carbon you see.
Two samples only from an unassessable time period with seventeen other contortions as to how large and how long the event was is evidence?
Look I’m a skeptic and I would not go there. Nor should you.
angech is trolling again “If you are prepared to set the limits then we can have a productive discussion in those limits, otherwise we have red (clay) herrings.”
this wasn’t at all a red herring, angech is just unable to accept that the only CaCO3 available to neutralise acid is the first 8cm or so of the sediment as the resulting non-calcerious clay isolates the remaining CaCo3 from the ocean. Shame on you angech for your behaviour.
“Two simple observations, the graphs show some severe loss of CaCO3 at some of 5 sites only at the PETM event time frame in one ocean only.”
of course if the whole ocean crust really was bathed in CaCO3 and the crust actually did have unlimited CaCO3 then what is shown in the third panel couldn’t have hapened even in one ocean. More disingenuous evasion from angech.
dikranmarsupial says:November 27, 2016 at 9:42 am
“angech is just unable to accept that the only CaCO3 available to neutralise acid is the first 8cm or so of the sediment as the resulting non-calcareous clay isolates the remaining CaCo3 from the ocean. Shame on you angech for your behavior.”
The ocean is supersaturated with CaCO3 in solution at the ocean surface and does not need to react with sediment in the first place.
I maintain that the earth/ ocean/ air complex that currently exists has had an extremely stable existence for 2 billion years and is quite resistant to severe pH change.
“The ultimate fate of much of the CO2 released to the atmosphere through the burning of coal, oil, and natural gas will be to react with the CaCO3 stored in marine sediments (Broecker and Takahashi, 1977; Sundquist, 1990; Archer et al., 1997).”
The geochemical control of seawater (Sillen revisited)
What he neglects to mention is that it also reacts with CaCO3 in the sea water producing CaHco3
Please remove above incomplete comment if possible moderator, hit post too early.
CO2 in Seawater: Equilibrium, Kinetics, Isotopes, 1st Edition Zeebe & Wolf-Gladrow have some wonderful stuff on our topic though hard to copy and paste.
It backs up ATTP and his presentation of equations.
The money line is page 41 1.2.4 total alkalinity and charge balance
“Sillen [1961] argued that considering the origin of the ocean, we might say that the ocean is the result of a gigantic acid base titration in which acids that have leached out from the interior of the earth are titrated with bases that have formed from the weathering of primary rock ”
–
That is the essence of what I have been saying.
In an authoritative text book.
–
Side tracking on permeability of sediments [undefined] and stating that the seafloor is covered with impermeable clay flies in the face of Broecker “The high-CaCO3 sediments that drape the oceans’ ridges and plateaus typically have ∼90% CaCO3 and a water-free density of 1 g cm−3.”
The amount of CaCO3 available for dissolution in such a sediment is 72 g cm−2.
This amount could neutralize 6.3×1017 mol of fossil fuel CO2. This amount exceeds the combined oceanic inventory of dissolved CO2− (1.6×1017 mol) and of dissolved VHBO3− (0.8×1017 mol). It is comparable to the amount of recoverable fossil fuel carbon.”
In other words the first 8cm or so of the available usable sediment
is not covered by impermeable clay, it is usable now.
It has the capacity to soak up 4 times the amount of CO2 already present in the ocean as DIC without blinking.
So it is available forever*, [* at least a very long time].
Only then would it be used up under an impermeable blanket as Dikran puts it.
It would be available to soak up all the fossil fuel that can theoretically be discovered.
I repeat, all the available fossil fuel ever.
And we still would not have an acid sea of consequence for the carbonic acid itself in equilibrium with the available CaCO3 is the buffer of the sea.
Your turn.
angech,
You seem to continually ignore that people do recognise that all of our emissions will eventually be soaked up. However, the timescale over which it will occur is about 100000 years.
“The ocean is supersaturated with CaCO3 in solution at the ocean surface and does not need to react with sediment in the first place.”
No it isn’t, I have already posted an abstract of a paper stating quite clearly that the abyssal ocean below the CCD is not supersaturated, and yet angech trots this out yet again.
I pointed out:
““angech is just unable to accept that the only CaCO3 available to neutralise acid is the first 8cm or so of the sediment as the resulting non-calcareous clay isolates the remaining CaCo3 from the ocean.”
angech says:
“The ocean is supersaturated with CaCO3 in solution at the ocean surface and does not need to react with sediment in the first place.
I maintain that the earth/ ocean/ air complex that currently exists has had an extremely stable existence for 2 billion years and is quite resistant to severe pH change.”
You will note that he STILL is unable to accept what is clearly stated by Archer and Broeker. This is just evasion to avoid admitting much of what he asserted upthread is factually incorrect.
“CO2 in Seawater: Equilibrium, Kinetics, Isotopes, 1st Edition Zeebe & Wolf-Gladrow have some wonderful stuff on our topic though hard to copy and paste.
It backs up ATTP and his presentation of equations.”
yes, I have a copy on the desk in front of me, however as far as I can see it says nothing whatsoever about the long term uptake of CO2 (being mostly concerned with CO2 in solution). Of course angech is free to give a page reference if he disagrees.
““Sillen [1961] argued that considering the origin of the ocean, we might say that the ocean is the result of a gigantic acid base titration in which acids that have leached out from the interior of the earth are titrated with bases that have formed from the weathering of primary rock ”
–
That is the essence of what I have been saying.
In an authoritative text book.”
no, it isn’t you are claiming considerably more than that, which is that there is sufficient CaC03 in the crust to neutralise any amount of acid (i.e. unlimited), which simply isn’t true as the quotes from Archer and Broeker (also authoritative sources) clearly state.
compare Borekers “It is comparable to the amount of recoverable fossil fuel carbon.”
with angech’s “It has the capacity to soak up 4 times the amount of CO2 already present in the ocean as DIC without blinking.”
no CaCO3 doesn’t neutralise DIC.
“Your turn.”
grow up.
That’s a misrepresentation of what I have written angech. You are going round in circles but it won’t help you with the fact that CO2 causes ocean acidification and marine ecosystem damage. I’ve posted on this thread about that as well, so addressing your false claim that I have avoided the question of what constitutes ‘severe’.
Don’t misrepresent me again, please. I’m allergic to it.
appologies, I misread this bit “The ocean is supersaturated with CaCO3 in solution at the ocean surface and does not need to react with sediment in the first place.”
in that case, one wonders why it is the part of the ocean where the pH is changing most rapidly? ;o)
dikranmarsupial says:
“The ocean is supersaturated with CaCO3 in solution at the ocean surface in that case, one wonders why it is the part of the ocean where the pH is changing most rapidly?”
It is at the interface where reacting with CO2 occurs, where changes in temperature vary the most both by night and day and by latitude , where evaporation occurs and where surface run off and air borne debris first arrives. Rhetorical question I guess.
Why does the pH go up as it approaches the bottom of the sea?
Non rhetorical.
You refuse to admit or consider this fact.
“You are going round in circles but it won’t help you with the fact that CO2 causes ocean acidification and marine ecosystem damage.”
Yes and no.
Yes, CO2 causes ocean acidification, in water it is a weak acid, it dissolves in water. yet the air contains 400 ppm and the pH is 8.2 so the ocean would be even more alkaline if the CO2 was not present. Presumably the acidification down to 8.2 is helping marine ecosystems.I do not think the ocean overall would like to be more alkaline.
PH in sea water varies extensively with depth and latitude and many marine lifeforms exist well in different pH zones, a change which you claim causes marine ecosystem damage in one area will make another marine system more alive and vibrant, it is not a one way damage only street.
It is so obvious, looking at one part of change only is just not right.
The level of CO2 in the air is normally due to the level of carbonate in the sea, not the other way round.
“Why does the pH go up as it approaches the bottom of the sea?”
I already explained that a few times, but you seem unwilling to read.
“Presumably the acidification down to 8.2 is helping marine ecosystems.”
How? If you make presumptions, you should have an explanation for that presumption
“I do not think the ocean overall would like to be more alkaline.”
Why not?
“PH in sea water varies extensively with depth and latitude and many marine lifeforms exist well in different pH zones, a change which you claim causes marine ecosystem damage in one area will make another marine system more alive and vibrant, it is not a one way damage only street.”
Please explain how e.g. tiny sea critters living in the tropical ocean surface can move to the arctic ocean surface, without changing LOADS of other ecosystems dependent on those sea critters being right there where they used to be before.
Seriously, if you complain about people not taking a global view, don’t ignore that global view yourself.
“The level of CO2 in the air is normally due to the level of carbonate in the sea, not the other way round.”
Please provide evidence for this claim.
angech wrote “It is at the interface where reacting with CO2 occurs, where changes in temperature vary the most both by night and day and by latitude , where evaporation occurs and where surface run off and air borne debris first arrives. Rhetorical question I guess.
Why does the pH go up as it approaches the bottom of the sea?”
The temperature, evaporation, surface run off and airborne debris arguments are invalid because they haven’t significantly changed (i.e. enough to make a change as large as that observed in ocean pH) over the period anthropogenic CO2 emissions have increased atmospheric CO2, which leaves the interface where reacting with CO2 occurs, however that brings us back to the start, where angech wrote:
“My contention is that the pH of the ocean exists in a reasonably narrow band and that concerns re rapid and dangerous pH changes in response to increasing CO2 rely on more than a simple surface exchange mechanism.”
so angech has contradicted himself yet again, which is because he is bullshitting. It wasn’t a rhetorical question, I just remember your own arguments better than you do.
“Why does the pH go up as it approaches the bottom of the sea?”
the explanation was given by Marco some time ago, but you ignored it, I didn’t
“PH in sea water varies extensively with depth and latitude and many marine lifeforms exist well in different pH zones, a change which you claim causes marine ecosystem damage in one area will make another marine system more alive and vibrant, it is not a one way damage only street.
I’ve already addressed that point more than once, and so has Marco. Ecosystems evolve to exploit a niche in the environment, often they become so adapted that they can’t tolerate even small changes in their environment (because the cost of tolerance is a reduce adaptation to exploit the niche). This means you can have organisms that are adapted to very different pH levels, but if you exchanged their environments they would both die. This is pretty basic evolution/ecology.
Some groups of humans, e.g. Tibetans have evolutionary adaptions that mean they can tolerate lower atmospheric oxygen. Your argument is a bit like saying that humans can survive oxygen levels 60% lower than at sea level, so we can all function normally at that altitude, but that simply isn’t the case.
Sorry, I’ve had enough of your bullshit. You may think you know the CaCO3/Carbon cycle better than David Archer and Wally Broeker, I don’t have that level of ridiculous hubris.
““The level of CO2 in the air is normally due to the level of carbonate in the sea, not the other way round.”
Please provide evidence for this claim.”
now there would be some truth to this if “normally” meant in pre-industrial conditions, but given we are discussing post-industrial ocean acidification, that argument is at best evasion.
… and only on geological timescales, of course!
Fluxes, a few figures may help
“The Earth’s Crust: The largest amount of carbon on Earth is stored in sedimentary rocks within the planet’s crust. These are rocks produced either by the hardening of mud (containing organic matter) into shale over geological time, or by the collection of calcium carbonate particles, from the shells and skeletons of marine organisms, into limestone and other carbon-containing sedimentary rocks. Together all sedimentary rocks on Earth store 100,000,000 PgC. Recalling that 1 Pg is over two trillion pounds, this is clearly a large mass of carbon! Another 4,000 PgC is stored in the Earth’s crust as hydrocarbons commonly known as fossil fuels.
Oceans: The Earth’s oceans contain 38,000 PgC, most of which is in the form of dissolved inorganic carbon stored at great depths where it resides for long periods of time. A much smaller amount of carbon, approximately 1,000 Pg, is located near the ocean surface.
Atmosphere: contains approximately 750 PgC, most of which is in the form of CO2,
Ocean—Atmosphere exchange: Inorganic carbon is absorbed and released at the interface of the oceans’ surface and surrounding air, through the process of diffusion. It may not seem obvious that gasses can be released from water”,
Most chemical reactions go from big to small. That is the larger amount dictates the smaller amount. Entropy.
Lol, we were talking about the availability of CaCO3 for moderating ocean acidity, none of the information that angech mentions has any bearing on that because once the carbonates in the top 8cm of the sediment are used up (and there is none below the CCD to start with), it isolates the rest from the ocean circulation. This has already been explained to angech, but he is still pretending it is not the limiting factor.
“Most chemical reactions go from big to small. That is the larger amount dictates the smaller amount. Entropy.”
On geological timescales it does (see the Archer paper), that doesn’t mean it dictates on short timescales to deal with spikes in atmospheric CO2 caused by anthropogenic emissions, therefore it is an irrelevant red-herring on human-relevant timescales.
“Atmosphere: contains approximately 750 PgC, most of which is in the form of CO2,
Ocean—Atmosphere exchange: Inorganic carbon is absorbed and released at the interface of the oceans’ surface and surrounding air, through the process of diffusion. It may not seem obvious that gasses can be released from water”,
just to be clear, are you arguing that the post industrial rise in atmospheric CO2 is caused by out-gassing of CO2 from the oceans? If so, you couldn’t be more wrong.
just to be clear, are you arguing that the post industrial rise in atmospheric CO2 is caused by out-gassing of CO2 from the oceans?
CO2 production by humans has gone up and adds to the post industrial rise in atmospheric CO2.
No one can tell if there is extra out-gassing of CO2 from the oceans from volcanic activity, we just do not have enough eyes on the deep seabed so I could not prove it is the cause.
There may also be increased biomass producing more CO2 as well.
I doubt that CaCo3 can contribute more DIC than it already does so I do not think that it is a cause of increasing CO2. Just a balance against excessive pH drop.
We need more data and more open minds.
However the CO2 in the atmosphere is in equilibrium with the DIC in the ocean.
If you removed CO2 from the atmosphere the ocean would just put more back into the air equivalent to the main source of CO2 in the atmosphere, the Carbonate in the ocean.
It cannot just sit there in the ocean without putting a large amount of CO2 into the air.
angech,
Sounds like you’re suggesting that the rise in atmospheric CO2 may not be anthropogenic. If you are, this is nonsense. It’s almost entirely anthropogenic. Of course, as CO2 is taken up by the oceans, and as we warm, the carbon sinks may behave differently, and – hence – influence the rise in atmospheric CO2, but that doesn’t suddenly make it not anthropogenic.
All comments positive and negative have encouraged me to look this up further on the internet. I know a lot more to argue with and to think about thanks to this. So while I am in disagreement with a lot of commentators here I am still learning and I hope you all are as well.
salient points are the CCD and its effects, thanks Dikran, and the paucity of information and knowledge on the crust formation and composition of the earth. Efforts to fit plate theory into this and the various pH of different bodies of water in the world. The complete dismissal of subterranean water and the lack of knowledge on how CaCO3 got into the ocean in the first place.
I hope some of my comments while rubbished at first and ongoing do make people think a little bit more about the complexity even if it does not change their views. I would not expect it to change their views on acidity in general anyway but understand some of the perspectives that are possible.
angech you have not unambiguously answered the question, why am I not surprised.
We know the rate at which atmospheric CO2 has been rising is less than the rate at which we are emitting CO2 into it. We know this with very high certainty due to the global network of stations at which atmospheric CO2 is measured and from financial/tax records of fossil fuel use, the uncertainty on which is far too small to affect this conclusion. That means the natural environment as a whole must be a net carbon sink, and has been taking more CO2 out of the atmosphere each year than it puts in. Do you agree with that, yes or no?
“It cannot just sit there in the ocean without putting a large amount of CO2 into the air.”
no this isn’t the case at all, the oceans and atmosphere were in dynamic equilibrium. If you disturb that equilibrium (e.g. by anthropogenic emissions into the atmosphere) then the fluxes will change to oppose that disturbance (if they didn’t there would be no equilibrium – this is basically Le Chatellier’s principle), so the net flux would be INTO the ocean, rather than out of it.
The PETM, angech, was not an ocean -> atmosphere flux of CO2. It went the other way around and the result was a significant shift in ocean pH. As people keep trying to explain to you.
Pingback: No, stabilising emissions will not stabilise concentrations! | …and Then There's Physics
Pingback: Residual airborne fraction | …and Then There's Physics
Pingback: Oh no, not again | …and Then There's Physics
Pingback: CO2 sequestration during glacial maxima | …and Then There's Physics